AmiFuse E
This treatment applies to the following species: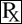 Company: Henry Schein® Animal Health
Company: Henry Schein® Animal Health
(Amikacin Sulfate Solution)
Description:
Amikacin sulfate is a semisynthetic aminoglycoside antibiotic derived from kanamycin. It is C22H43N5O13•2H2SO4,D-streptamine, 0 - 3 - amino - 3 - deoxy - α - D - glucopyranosyl - (1 - 6) - 0 - [6 - amino - 6 - deoxy - α - D - glucopyranosyl - (1 - 4) - N1 - (4 - amino - 2 - hydroxy - 1 - oxobutyl)-2-deoxy-, (S)-, sulfate (1:2)(salt).
The dosage form supplied is a sterile, colorless to light straw colored solution. The solution contains, in addition to amikacin sulfate, 2.5% sodium citrate with pH adjusted to 4.5 with sulfuric acid and 0.66% sodium bisulfite added. The multi-dose 12 gram vial contains 0.01% benzethonium chloride as a preservative.
ACTION: Antibacterial Activity
The effectiveness of AmiFuse E (Amikacin Sulfate Solution) in infections caused by Escherichia coli, Pseudomonas sp, and Klebsiella sp has been demonstrated clinically in the horse. In addition, the following microorganisms have been shown to be susceptible to amikacin in vitro(1), although the clinical significance of this action has not been demonstrated in animals:
Enterobacter sp, Proteus mirabilis, Proteus sp (indole positive), Serratia marcescens, Salmonella sp, Shigella sp, Providencia sp, Citrobacter freundii, Listeria monocytogenes Staphylococcus aureus (both penicillin resistant and penicillin sensitive).
The aminoglycoside antibiotics in general have limited activity against gram-positive pathogens, although Staphylococcus aureus and Listeria monocytogenes are susceptible to amikacin as noted above.
Amikacin has been shown to be effective against many aminoglycoside-resistant strains due to its ability to resist degradation by aminoglycoside inactivating enzymes known to affect gentamicin, tobramycin and kanamycin(2).
Clinical Pharmacology:
Endometrial Tissue Concentrations
Comparisons of amikacin activity in endometrial biopsy tissue following intrauterine infusion with that following intramuscular injection of amikacin sulfate in mares demonstrate superior endometrial tissue concentrations when the drug is administered by the intrauterine route. Intrauterine infusion of 2 grams AmiFuse E (Amikacin Sulfate Solution) daily for three consecutive days in mares results in peak concentrations typically exceeding 40 mcg/g of endometrial biopsy tissue within one hour after infusion. Twenty-four hours after each treatment amikacin activity is still detectable at concentrations averaging 2 to 4 mcg/g. However, the drug is not appreciably absorbed systemically following intrauterine infusion. Endometrial tissue concentrations following intramuscular injection roughly parallel, but are typically somewhat lower than corresponding serum concentrations of amikacin.
Safety:
AmiFuse E (Amikacin Sulfate Solution) is non-irritating to equine endometrial tissue when infused into the uterus as directed (see “Dosage and Administration”). In laboratory animals as well as equine studies, the drug was generally found not to be irritating when injected intravenously, subcutaneously or intramuscularly.
Although amikacin, like other aminoglycosides, is potentially nephrotoxic, ototoxic and neurotoxic, parenteral (intravenous) administration of amikacin sulfate twice daily at dosages of up to 10 mg/lb for 15 consecutive days in horses resulted in no clinical, laboratory or histopathologic evidence of toxicity.
Intrauterine infusion of 2 grams of AmiFuse E (Amikacin Sulfate Solution) 8 hours prior to breeding by natural service did not impair fertility in mares. Therefore, mares should not be bred for at least 8 hours following uterine infusion.
AmiFuse E Indications:
AmiFuse E (Amikacin Sulfate Solution) is indicated for the treatment of uterine infections (endometritis, metritis and pyometra) in mares, when caused by susceptible organisms including Escherichia coli, Pseudomonas sp, and Klebsiella sp. The use of amikacin sulfate in eliminating infections caused by the above organisms has been shown clinically to improve fertility in infected mares.
While nearly all strains of Escherichia coli, Pseudomonas sp and Klebsiella sp, including those that are resistant to gentamicin, kanamycin or other aminoglycosides, are susceptible to amikacin at levels achieved following treatment, it is recommended that the invading organism be cultured and its susceptibility demonstrated as a guide to therapy. Amikacin susceptibility discs, 30 mcg, should be used for determining in vitro susceptibility.
AmiFuse E Dosage And Administration:
For treatment of uterine infections in mares, 2 grams (8 mL) of AmiFuse E (Amikacin Sulfate Solution), mixed with 200 mL 0.9% Sodium Chloride Injection, USP and aseptically infused into the uterus daily for three consecutive days, has been found to be the most efficacious dosage.
Contraindications:
There are no known contraindications for the use of amikacin sulfate in horses other than a history of hypersensitivity to amikacin.
Precautions:
Although amikacin sulfate is not absorbed to an appreciable extent following intrauterine infusion, concurrent use of other aminoglycosides should be avoided because of the potential additive effects.
Adverse Reactions:
No adverse reactions or other side effects have been reported.
Warning:
Not to be used in horses intended for food. In vitro studies have demonstrated that when sperm are exposed to the preservative which is present in the 48 mL vials (250 mg/mL) sperm viability is impaired.
AmiFuse E Caution:
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Supply:
AmiFuse E (Amikacin Sulfate Solution) is supplied as a colorless solution which is stable at room temperature. At times the solution may become pale yellow in color. This does not indicate a decrease in potency.
48 mL vial, 250mg/mL
Store at controlled room temperature 15°-30°C (59°-86°F).
References
1. Price, K.E., et al: Microbiological Evaluation of BB-K8, a New Semisynthetic Aminoglycoside. J. Antibiot. 25: 709-731, 1972.
2. Davies, J., Courvalin, P.: Mechanism of Resistance to Aminoglycosides. Am J Med 62: 868-872, 1977.
ANADA 200-181, Approved by FDA
CPN: 10822412
400 METRO PLACE NORTH, DUBLIN, OH, 43017-7545
| Telephone: | 614-761-9095 | |
| Toll-Free: | 1-855-724-3461 |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
