Dtap Vaccine for Children
Medically reviewed by Drugs.com. Last updated on Aug 4, 2025.
What is the DTaP vaccine?
DTaP is a vaccine given as a shot to protect your child from diphtheria, tetanus, and pertussis (whooping cough). These are severe infections caused by bacteria. Tetanus bacteria are found in dirt, manure, and dust. The bacteria enter the body through open skin, such as puncture wounds and burns. Diphtheria and pertussis bacteria are spread from person to person. The DTaP vaccine will protect your child until about age 11. Then he or she will need booster shots.
When is the DTaP vaccine given?
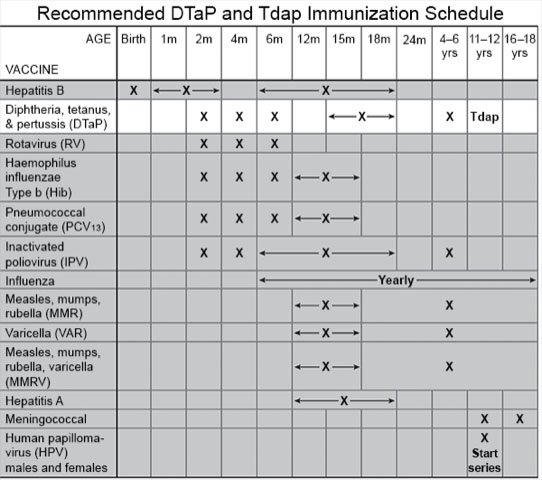
The DTaP vaccine is only given to children younger than 7 years, starting as early as 6 weeks. Children usually get 4 or 5 doses of the DTaP vaccine. If your child misses a dose, the next dose should be given as soon as possible. Your child will not need extra doses or start the entire series again. A dose is sometimes given to children younger than 7 years after a severe wound or burn to prevent tetanus. The DTaP vaccine is often given with polio, hepatitis B, pneumococcal, and Hib vaccines. Your child may need these or other childhood vaccines at certain ages. DTaP is usually given at the following ages:
- The first dose at 2 months
- The second dose at 4 months
- The third dose at 6 months
- The fourth dose at 15 to 18 months
- The fifth dose at 4 to 6 years if the fourth dose was given before age 4
 |
What do I need to tell my child's healthcare provider?
Tell the provider if your child has or had any of the following:
- Infantile spasms, a seizure disorder that is not controlled, or a brain disorder that is not stable
- Any severe allergy
- A seizure or collapse after a dose of DTaP
- Guillain-Barré syndrome that developed less than 6 weeks after a dose of DTaP
- A hypersensitivity reaction to a dose of DTaP
- A period of crying for more than 3 hours within the first 2 days of a DTaP dose
- A fever of 105º F (40.5º C) after a dose of DTaP
What are reasons my child should not get the DTaP vaccine?
Your child should not get the vaccine if he or she has or had any of the following:
- An allergy to any part of the vaccine
- A severe allergic reaction to a dose of DTaP
- Seizures or a coma within 7 days of a dose, caused by the DTaP vaccine
Related medications
When should my child wait to get the DTaP vaccine?
Tell your child's healthcare provider if your child has a fever or illness. Your child's provider may wait to give the DTaP vaccine until the fever or illness is gone.
What are the risks of the DTaP vaccine?
The area where the vaccine was given may be red, tender, or swollen. This should get better in 1 to 2 days. Rarely, your child may develop severe shoulder pain that lasts longer than 2 days. Your child may have an allergic reaction to the vaccine. Rarely, this can be life-threatening.
Call your local emergency number (911 in the US) if:
- Your child has signs of a severe allergic reaction, such as trouble breathing, hives, or wheezing.
- Your child begins to have seizures (staring or jerking).
- Your child has a fever of 105º F (40.5º C).
When should I call my child's doctor?
- Your child cries for 3 or more hours after getting DTaP.
- Your child's face is red or swollen.
- Your child has hives that spread over his or her body.
- Your child feels weak or dizzy.
- Your child has a headache, body aches, or joint pain.
- Your child has nausea or diarrhea, or he or she is vomiting.
- You have questions or concerns about the vaccine.
Care Agreement
You have the right to help plan your child's care. Learn about your child's health condition and how it may be treated. Discuss treatment options with your child's healthcare providers to decide what care you want for your child. The above information is an educational aid only. It is not intended as medical advice for individual conditions or treatments. Talk to your doctor, nurse or pharmacist before following any medical regimen to see if it is safe and effective for you.© Copyright Merative 2025 Information is for End User's use only and may not be sold, redistributed or otherwise used for commercial purposes.
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
