Umecta Mousse: Package Insert / Prescribing Info
Package insert / product label
Generic name: urea
Dosage form: aerosol, foam
Drug class: Topical emollients
Medically reviewed by Drugs.com. Last updated on Mar 3, 2025.
On This Page
Umecta Mousse Description
Rx only
For topical use only
Not for ophthalmic use
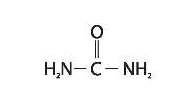
Umecta is a keratolytic, emollient which is a gentle, yet potent, tissue softener for nails and skin Each gram of Umecta mousse contains urea (40%), Butane, Butyrospermum Parkii (Shea Butter) Extract, Carbomer, Glycine Soya (Soy Bean) Sterol, Helianthus Annuus (Sunflower) Oil, Isobutane, Laureth-4, Polysorbate-20, Propane, Purified Water, Stearic Acid, Triethanolamine. Urea is a diamide of carbonic acid with the following chemical structure:
Umecta Mousse - Clinical Pharmacology
Urea gently dissolves the intercellular matrix which results in loosening the horny layer of skin and shedding scaly skin at regular intervals, thereby softening hyperkeratotic areas. Urea also hydrates and gently dissolves the intercellular matrix of the nail plate which can result in the softening and eventual debridement of the nail plate.
Indications and Usage for Umecta Mousse
For debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris or eschar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis, keratoderma, corns, and calluses, as well as damaged, ingrown and devitalized nails.
Warnings
For external use only. Avoid contact with eyes, lips or mucous membranes. Umecta Mousse canister - contents under pressure do not puncture or incinerate. Do not store at temperatures above 120º F.
Precautions
This medication is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use.
Pregnancy
Animal reproduction studies have not been conducted with Umecta. It is also not known whether Umecta can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Umecta should be given to a pregnant woman only if clearly needed.
Nursing Monthers
It is not known whether or not this drug is secreted in human milk. Because many drugs are secreted in human milk, caution should be exercised when Umecta is administered to a nursing woman.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Adverse Reactions/Side Effects
Transient stinging, burning, itching, or irritation may occur and normally disappear on discontinuing the medication.
Related/similar drugs
Umecta Mousse Dosage and Administration
Apply Umecta PD bioadhesive emulsion/topical suspension or Umecta mousse to affected skin twice per day, or as directed by a physician. Rub in until completely absorbed
| UMECTA MOUSSE
UREA
urea foam aerosol, foam |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - EPI Health, Inc (080638894) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| EPI Health, Inc | 080638894 | manufacture(71403-020) | |

