MeTopic Cream: Package Insert / Prescribing Info
Package insert / product label
Generic name: urea
Dosage form: cream
Medically reviewed by Drugs.com. Last updated on Mar 31, 2025.
The MeTopic brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
On This Page
NDC 58657-485-08
MeTopic Cream
(Urea 41%)
For External Use Only Rx Only
Net Wt. 8 oz. (227 g
MeTopic
Method Pharmaceuticals, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA.
----------
MeTopic Cream DESCRIPTION:
Each gram contains 410 mg of urea in a vehicle consisting of: ceteareth-25, ceteareth-6, cetyl alcohol, methylparaben, paraffin, propylene glycol, propylparaben, purified water, stearyl alcohol, xanthan gum.
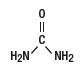
Urea is a diamide of carbonic acid with the following chemical structure:
MeTopic Cream - Clinical Pharmacology
Urea gently dissolves the intercellular matrix which results in loosening the horny layer of skin and shedding scaly skin at regular intervals, thereby softening hyperkeratotic areas of the skin.
Pharmacokinetics: The mechanism of action of topically applied urea is not yet known.
Indications and Usage for MeTopic Cream
This product is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, xerosis, ichthyosis, skin cracks and fissures, dermatitis, eczema, psoriasis, keratoses and calluses.
Contraindications
This product is contraindicated in persons with known or suspected hypersensitivity to any of the ingredients of the product.
Warnings
FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE. KEEP OUT OF REACH OF CHILDREN.
Avoid contact with eyes, lips and mucous membranes.
General:
This product is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use and consult a physician.
Information for Patients:
Patients should discontinue the use of this product if the condition becomes worse or if a rash develops in the area being treated or elsewhere. Avoid contact with eyes, lips and mucous membranes.
Carcinogenesis, Mutagenesis and Impairment of Fertility:
Long-term animal studies for carcinogenic potential have not been performed on this product to date. Studies on reproduction and fertility also have not been performed.
Pregnancy:
Category C. Animal reproduction studies have not been conducted with this product. It is also not known whether this product can affect reproduction capacity or cause fetal harm when administered to a pregnant woman. This product should be used by a pregnant woman only if clearly needed or when potential benefits outweigh potential hazards to the fetus.
Adverse Reactions/Side Effects
Transient stinging, burning, itching or irritation may occur and normally disappear upon discontinuing the use of this product.
Related/similar drugs
MeTopic Cream Dosage and Administration
Apply to affected area(s) twice per day or as directed by a physician. Rub in until completely absorbed.
See package insert for full prescribing information.
STORAGE:
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C to 30°C (between 59°F to 86°F). Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimized.
NOTICE: Protect from freezing and excessive heat. Keep bottle tightly closed.
How is MeTopic Cream supplied
This product is supplied in the following size(s): 8 oz. (227 g) bottles, NDC # 58657-485-08
To report a serious adverse event or obtain product information, call 877-250-3427
To report a serious adverse event, please contact Method Pharmaceuticals at (877) 250-3427; email at contact@methodpharm.com; or call FDA at (800) FDA-1088.
Manufactured for:
Method Pharmaceuticals, LLC Fort Worth, Texas 76118
Rev. 03/18
| METOPIC
urea cream |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Method Pharmaceuticals, LLC (060216698) |
More about MeTopic (urea topical)
Professional resources
Other brands
Keralac, Rynoderm, Umecta Mousse, URE-39, URE-K



