Twyneo: Package Insert / Prescribing Info
Package insert / product label
Generic name: tretinoin and benzoyl peroxide
Dosage form: cream
Drug class: Topical acne agents
Medically reviewed by Drugs.com. Last updated on Aug 17, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
TWYNEO® (tretinoin and benzoyl peroxide) cream, 0.1%/3%, for topical use.
Initial U.S. Approval: 2021
Indications and Usage for Twyneo
TWYNEO is a combination tretinoin, a retinoid, and benzoyl peroxide indicated for the topical treatment of acne vulgaris in adults and pediatric patients 9 years of age and older. (1)
Twyneo Dosage and Administration
Dosage Forms and Strengths
Cream, 0.1% tretinoin/3% benzoyl peroxide (3)
Contraindications
History of serious hypersensitivity reaction to benzoyl peroxide or any component of TWYNEO. (4)
Warnings and Precautions
- Hypersensitivity: Severe hypersensitivity reactions, including anaphylaxis and angioedema, have been reported with use of benzoyl peroxide products. (4, 5.1)
- Skin Irritation: Pain, dryness, exfoliation, erythema, and irritation may occur with use of TWYNEO. Avoid application of TWYNEO to cuts abrasions, eczematous or sunburned skin. (5.2)
- Photosensitivity: Minimize unprotected exposure to sunlight and sunlamps. Use sunscreen and protective clothing when sun exposure cannot be avoided. (5.3)
Adverse Reactions/Side Effects
The most common adverse reactions (incidence ≥ 1%) are pain, dryness, exfoliation erythema, dermatitis, pruritus and irritation (all at the application site). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma. at 1-844-825-8500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2025
Full Prescribing Information
1. Indications and Usage for Twyneo
TWYNEO is indicated for the topical treatment of acne vulgaris in adults and pediatric patients 9 years of age and older.
2. Twyneo Dosage and Administration
- Apply a thin layer of TWYNEO to the affected areas once daily on clean and dry skin. Avoid contact with the eyes, lips, paranasal creases, and mucous membranes.
- Wash hands after application.
- TWYNEO is for topical use only. TWYNEO is not for oral, ophthalmic, or intravaginal use.
3. Dosage Forms and Strengths
Cream, 0.1%/3%: Each gram of TWYNEO contains 1mg (0.1%) of tretinoin and 30 mg (3%) of benzoyl peroxide in a yellow cream in a 30-gram bottle with a pump.
4. Contraindications
TWYNEO is contraindicated in patients with a history of hypersensitivity reaction to benzoyl peroxide or any components of TWYNEO [see Warnings and Precautions (5.1)].
5. Warnings and Precautions
5.1 Hypersensitivity
Hypersensitivity reactions, including anaphylaxis, angioedema, and urticaria, have been reported with the use of benzoyl peroxide products. If a serious hypersensitivity reaction occurs, discontinue TWYNEO immediately and initiate appropriate therapy.
5.2 Skin Irritation
Patients using TWYNEO may experience application site dryness, pain, exfoliation, erythema, dermatitis, pruritis, and irritation [see Adverse Reactions (6.1)]. Depending upon the severity of these adverse reactions, instruct patients to use a moisturizer, reduce the frequency of the application of TWYNEO, or discontinue use. Avoid application of TWYNEO to cuts, abrasions, eczematous, or sunburned skin.
5.3 Photosensitivity
TWYNEO may increase sensitivity to ultraviolet light. Minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using TWYNEO. Instruct patients to implement sun protection measures (e.g., sunscreen and loose- fitting clothes) when sun exposure cannot be avoided. Discontinue TWYNEO at the first evidence of sunburn.
6. Adverse Reactions/Side Effects
6.1 Clinical trials experience
The following adverse reactions are discussed in greater detail elsewhere in the labeling:
- Hypersensitivity [see Warnings and Precautions (5.1)]
- Skin Irritation [see Warnings and Precautions (5.2)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates are observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In two multicenter, randomized, double-blind, vehicle-controlled trials (Trial 1 and 2), 832 subjects 9 years of age and older with facial acne vulgaris applied TWYNEO (N=555) or vehicle (N=277) daily for 12 weeks. The majority of subjects were White (73%) and female (59%).
Approximately 33% were Hispanic/Latino, and 46% were younger than 18 years of age. Adverse reactions reported in ≥ 1.0% of subjects treated with TWYNEO (and for which the rate exceeded the rate for vehicle), as well as the corresponding rates reported in subjects treated with vehicle are presented in Table 1.
| TWYNEO Cream (N = 555) n (%) | Vehicle Cream (N = 277) n (%) |
|
|---|---|---|
|
||
| Application Site Pain* | 59 (10.6) | 1 (0.4) |
| Application Site Dryness | 27 (4.9) | 1 (0.4) |
| Application Site Exfoliation | 23 (4.1) | 0 |
| Application Site Erythema | 22 (4.0) | 0 |
| Application Site Dermatitis | 7 (1.3) | 1 (0.4) |
| Application Site Pruritus | 7 (1.3) | 0 |
| Application Site Irritation | 6 (1.1) | 1 (0.4) |
Local tolerability evaluations were conducted at each study visit in the clinical trial by assessment of erythema, scaling, pigmentation, dryness, itching, burning, and stinging. Table 2 presents the active assessment of the signs and symptoms of local facial tolerability at Week 12 in subjects treated with TWYNEO.
| TWYNEO (N=494*) (%) | Vehicle (N = 264*) (%) |
|||||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | |
|
||||||
| Erythema | 33.0 | 6.9 | 0.2 | 26.9 | 8.0 | 0 |
| Pigmentation | 27.3 | 6.3 | 0.4 | 26.5 | 4.5 | 0 |
| Dryness | 22.3 | 5.3 | 0.4 | 16.7 | 2.3 | 0 |
| Scaling | 16.4 | 2.6 | 0 | 12.9 | 0.8 | 0 |
| Burning | 5.9 | 2.2 | 0 | 3.4 | 0.8 | 0 |
| Itching | 11.1 | 1.8 | 0 | 8.7 | 2.7 | 0 |
| Stinging | 5.3 | 0.2 | 0 | 1.9 | 1.1 | 0 |
Local tolerability scores for erythema, scaling, dryness, itching, burning, and stinging rose during the first two weeks of treatment and decreased thereafter.
6.2 Postmarketing Experience
The following adverse reactions have been identified during use of benzoyl peroxide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Anaphylaxis, angioedema and urticaria
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Available data from published observational studies of topical tretinoin in pregnant women have not established a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Studies conducted with topical benzoyl peroxide have not demonstrated systemic absorption and maternal use is not expected to result in fetal exposure to benzoyl peroxide. There are no data on TWYNEO use in pregnant women.
There are reports of major birth defects reported with maternal use of topical tretinoin similar to those seen in infants exposed to oral retinoids, but these case reports do not establish a pattern or association with tretinoin-related embryopathy (see Data).
Animal reproductive studies have not been conducted with TWYNEO or benzoyl peroxide. Topical administration of tretinoin to pregnant rats during organogenesis was associated with malformations (craniofacial abnormalities [hydrocephaly], asymmetrical thyroids, variations in ossification, and increased supernumerary ribs) at doses greater than 1 mg tretinoin/kg/day, approximately 5 times the maximum recommended human dose (MRHD) based on body surface area (BSA) comparison and assuming 100% absorption. Oral administration of tretinoin to pregnant cynomolgus monkeys during organogenesis was associated with malformations at 10 mg/kg/day (approximately 100 times the MRHD based on BSA comparison and assuming 100% absorption) (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of major birth defects, loss and other adverse outcomes. The background risk in the U.S. general population of major birth defects is 2 to 4% of miscarriage is 15 to 20% of clinically recognized pregnancies.
Data
Human Data
While available studies cannot definitively establish the absence of risk, published data from multiple prospective controlled observational studies on the use of topical tretinoin products during pregnancy have not identified an association with topical tretinoin and major birth defects or miscarriage. The available studies have methodologic limitations, including small sample size and in some cases, lack of physical exam by an expert in birth defects. There are published case reports of infants exposed to topical tretinoin during the first trimester that describe major birth defects similar to those seen in infants exposed to oral retinoids; however, no pattern of malformations has been identified and no causal association has been established in these cases. The significance of these spontaneous reports in terms of risk to the fetus is not known.
Animal Data
For purposes of comparison of the animal exposure to human exposure, the MRHD is defined as 1.5 g of TWYNEO (containing 0.1% tretinoin) applied daily to a 60-kg person (0.03 mg tretinoin/kg body weight).
Topical tretinoin embryofetal development studies have generated equivocal results. There is evidence for malformations (shortened or kinked tail) after topical tretinoin administration in Wistar rats at doses greater than 1 mg/kg/day (approximately 5 times the MHRD based on BSA comparison and assuming 100% absorption). Anomalies (humerus: short 13%, bent 6%, or parietal incompletely ossified 14%) have also been reported when 10 mg/kg/day (approximately 50 times the MRHD based on BSA comparison and assuming 100% absorption) was topically applied to pregnant rats during organogenesis. Increased incidence of domed head and hydrocephaly, typical of retinoid-induced fetal malformations were noted in New Zealand White rabbits administered topical doses greater than 0.2 mg/kg/day (2.2 times the MRHD based on BSA comparison and assuming 100% absorption).
Oral tretinoin induced malformations in rats, mice, hamsters, and nonhuman primates when administered during the period of organogenesis. Fetal malformations were observed when tretinoin was orally administered to pregnant Wistar rats during organogenesis. It was teratogenic and fetotoxic in Wistar rats when given orally or topically in doses greater than 1 mg/kg/day (approximately 5 times the MRHD based on BSA comparison and assuming 100% absorption). In the cynomolgus monkey, fetal malformations were reported when an oral dose of 10 mg/kg/day was administered to pregnant monkeys during organogenesis (approximately 100 times the MRHD based on BSA comparison and assuming 100% absorption). No fetal malformations were observed at an oral dose of 5 mg/kg/day (approximately 50 times the MRHD based on BSA comparison and assuming 100% absorption). Increased skeletal variations were observed at all doses, and a dose-related increase in embryo lethality and abortion was reported in this study. Similar results have also been reported in pigtail macaques. Oral tretinoin has been shown to be fetotoxic in rats when administered at a dose of 2.5 mg/kg/day (13 times the MRHD based on BSA comparison and assuming 100% absorption). Topical tretinoin has been shown to be fetotoxic in rabbits when administered at a dose of 0.5 mg/kg/day (5 times the MRHD based on BSA comparison and assuming 100% absorption).
8.2 Lactation
Risk Summary
There are no data on the presence of benzoyl peroxide and tretinoin or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. It is not known whether topical administration of tretinoin could result in sufficient systemic absorption to produce detectable concentrations in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for TWYNEO and any potential adverse effects on the breastfed child from TWYNEO or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of TWYNEO for the topical treatment of acne vulgaris have been established in pediatric patients 9 years of age and older based on evidence from two multicenter, randomized, double-blind, parallel-group, vehicle-controlled, 12-week clinical trials and an open-label pharmacokinetic study. A total of 283 pediatric subjects 9 years of age and older received TWYNEO in the clinical studies [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
The safety and effectiveness of TWYNEO in pediatric patients below 9 years of age have not been established.
11. Twyneo Description
TWYNEO (tretinoin and benzoyl peroxide) cream is a yellow cream for topical use. Each gram of TWYNEO contains 1 mg (0.1%) of tretinoin and 30 mg (3%) of benzoyl peroxide. Tretinoin is a retinoid and benzoyl peroxide is an oxidizing agent.
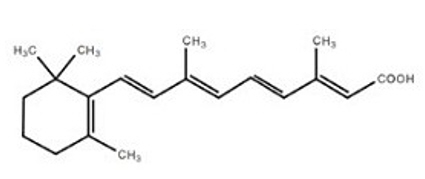
The chemical name for tretinoin is all-trans-retinoic acid, also known as (all-E)-3,7- dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid. Tretinoin has the following structural formula:
 |
|
| Molecular Formula: C20H28O2 | Molecular Weight: 300.44 |
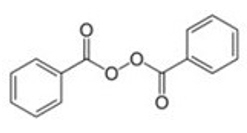
The chemical name for benzoyl peroxide is benzoyl benzenecarboperoxoate. Benzoyl peroxide has the following structural formula:
 |
|
| Molecular Formula: C14H10O4 | Molecular Weight: 242.23 |
The formulation of TWYNEO uses silica (silicon dioxide) core shell structures to separately micro-encapsulate tretinoin crystals and benzoyl peroxide crystals enabling inclusion of the two active ingredients in the. TWYNEO contains the following inactive ingredients: anhydrous citric acid, butylated hydroxytoluene, carbomer homopolymer type C, cetrimonium chloride, cetyl alcohol, cyclomethicone, edetate disodium, glycerin, hydrochloric acid, imidurea, (S)lactic acid, macrogol stearate, mono and di-glycerides, polyquaternium-7, purified water, silicon dioxide, sodium hydroxide, squalane, tetraethyl ortho silicate and white wax.
12. Twyneo - Clinical Pharmacology
12.1 Mechanism of Action
Benzoyl peroxide is an oxidizing agent with bactericidal and keratolytic effects, but the precise mechanism of action is unknown. Tretinoin is a metabolite of vitamin A that binds with high affinity to specific retinoic acid receptors located in both the cytosol and nucleus. Tretinoin activates three members of the retinoic acid (RAR) nuclear receptors (RARα , RARβ, RARγ) which act to modify gene expression, subsequent protein synthesis, and epithelial cell growth and differentiation. It has not been established whether the clinical effects of tretinoin are mediated through activation of retinoic acid receptors, and/or other mechanisms.
Although the exact mode of action of tretinoin in acne treatment is unknown, current evidence suggests that topical tretinoin decreases cohesiveness of follicular epithelial cells with decreased microcomedo formation. Additionally, tretinoin stimulates mitotic activity and increased turnover of follicular epithelial cells causing extrusion of the comedones.
12.3 Pharmacokinetics
The systemic exposure of benzoyl peroxide was not assessed. Benzoyl peroxide is absorbed by skin where it is converted to benzoic acid and eliminated in the urine.
Plasma concentrations of tretinoin and its major metabolites were evaluated in 35 subjects in an open-label, randomized, pharmacokinetic (PK) study. Subjects 9 years of age and older with acne vulgaris applied a mean dose of 1.9 g TWYNEO to the skin of the face, shoulders, upper back and upper chest once daily for 14 days.
Steady-state PK characteristics were determined from samples drawn on Day 14. The mean baseline corrected Cmax and AUC0-24 of tretinoin and its metabolites after once daily application of TWYNEO for 14 days are provided in Table 3. No detectable levels of the metabolites a l-trans 4-keto retinoic acid and 9-cis retinoid acid were found in subjects treated with TWYNEO.
| Age Group (years) | n | Compound | Mean(± SD) Cmax (ng/mL) | Mean(± SD) AUC0-24 (ng-h/mL) |
|---|---|---|---|---|
| ≥ 18 years of age | 12 | tretinoin | 0.15 ± 0.17 | 0.63 ± 0.95 |
| 4-keto 13-cis RA | 0.27 ± 0.29 | 2.88 ± 3.61 | ||
| 13-cis RA | 0.21 ± 0.19 | 1.99 ± 2.90 | ||
| 12 to 17 | 15 | tretinoin | 0.19 ± 0.18 | 1.56 ± 1.97 |
| 4-keto 13-cis RA | 0.32 ± 0.28 | 2.39 ± 3.05 | ||
| 13-cis RA | 0.28 ± 0.35 | 1.79 ± 2.79 | ||
| 9 to 11 | 8 | tretinoin | 0.18 ± 0.22 | 2.06 ± 3.96 |
| 4-keto 13-cis RA | 0.34 ± 0.36 | 2.89 ± 3.17 | ||
| 13-cis RA | 0.13 ± 0.09 | 0.96 ± 1.36 |
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity, mutagenicity, and impairment of fertility studies were not conducted with TWYNEO.
Benzoyl peroxide
The role of benzoyl peroxide as a tumor promoter has been well established in several animal species; however the significance of this finding in humans is unknown.
No significant increase in tumor formation was observed in rats treated topically with 15 to 25% benzoyl peroxide carbopol gel (5 to 8 times the concentration of benzoyl peroxide in TWYNEO) for two years. Similar results were obtained in mice topically treated with 25% benzoyl peroxide carbopol gel for 56 weeks followed by intermittent treatment with 15% benzoyl peroxide gel for the rest of the two-year study period, and in mice treated topically with 5% benzoyl peroxide carbopol gel for two years.
Bacterial mutagenicity assays (Ames test) conducted with benzoyl peroxide have provided mixed results. Mutagenic potential was observed in a few studies but not in a majority of investigations. Benzoyl peroxide has been found to cause DNA strand breaks in a variety of mammalian ce l types and to cause sister chromatid exchanges in Chinese hamster ovary cells.
Fertility studies were not conducted with benzoyl peroxide.
Tretinoin
In a 91-week dermal study, CD-1 mice were administered 0.017% and 0.035% formulations of tretinoin, cutaneous squamous cell carcinomas and papillomas in the treatment area were observed in some female mice. A dose-related incidence of liver tumors in male mice was observed at those same doses. The maximum systemic doses associated with the administered 0.017% and 0.035% formulations are 0.5 and 1.0 mg/kg/day, respectively. These doses are 1.3 and 2.7 times the MRHD based on BSA comparison and assuming 100% absorption. The biological significance of these findings is not clear because they occurred at doses that exceeded the dermal maximally tolerated dose (MTD) of tretinoin and because they were within the background natural occurrence rate for these tumors in this strain of mice. There was no evidence of carcinogenic potential when 0.025 mg/kg/day of tretinoin was administered topically to mice (0.07 times the MRHD based on BSA comparison and assuming 100% absorption). The genotoxic potential of tretinoin was evaluated in an in vitro bacterial reversion test and an in vivo rat micronucleus assay, both of which were negative.
In dermal fertility studies of another tretinoin formulation in rats, slight (not statistically significant) decreases in sperm count and motility were seen at 0.5 mg/kg/day (approximately 2.7 times the MRHD based on BSA comparison and assuming 100% absorption), and slight (not statistically significant) increases in the number and percent of nonviable embryos in females treated with 0.25 mg/kg/day and above (1.3 times MRHD based on BSA comparison and assuming 100% absorption) were observed.
14. Clinical Studies
The safety and efficacy of TWYNEO was evaluated in the treatment of acne vulgaris in two multicenter, randomized, double-blind, vehicle-controlled trials [Trial 1 (NCT03761784), Trial 2 (NCT03761810)], which were identical in design. The trials were conducted in 858 subjects 9 years of age and older, with facial acne vulgaris who were treated once daily for 12 weeks with either TWYNEO or vehicle.
Subjects were required to have a score of moderate (3) or severe (4) on the Investigator Global Assessment (IGA), 20 to 100 inflammatory lesions (papules, pustules and nodules), 30 to 150 non-inflammatory lesions (open and closed comedones) and two or fewer facial nodules. Overall, 73% of subjects were White and 59% were female. Eighteen (18) (2%) subjects were 9 to 11 years of age, 370 (43%) subjects were 12 to 17 years of age, and 470 (55%) subjects were 18 years of age or older. At baseline, subjects had a mean inflammatory lesion count of 30.7 and a mean noninflammatory lesion count of 46.4.
Additionally, 91% of subjects had an IGA score of 3 ("moderate").
The co-primary efficacy endpoints were the absolute change from baseline in non- inflammatory lesion count, and absolute change in inflammatory lesion count at Week 12 and the proportion of subjects with IGA success at Week 12, defined as an IGA score of 0 ("clear") or 1 ("almost clear"), and at least a two-grade improvement (decrease) from baseline at Week 12. The efficacy results are provided in Table 4.
| Trial 1 | Trial 2 | |||
|---|---|---|---|---|
| TWYNEO (N = 281) | Vehicle N = 143) | TWYNEO (N = 290) | Vehicle (N = 144) |
|
| IGA Success* | 33.9% | 14.3% | 26.8% | 15.1% |
| Difference from Vehicle | 25.7% | 11.6% | ||
| (95% CI) | (17.1%, 34.2%) | (3.6%, 19.7%) | ||
| Inflammatory Lesions | ||||
| Mean† Absolute Change from Baseline | -21.6 | -14.8 | -16.2 | -14.1 |
| Difference from Vehicle | -6.8 | -2.1 | ||
| (95% CI) | (-9.1, -4.6) | (-3.9, -0.4) | ||
| Mean† Percent Change from Baseline | -66.1% | -43.5% | -57.6% | -50.8% |
| Difference from Vehicle | -22.6% | -6.8% | ||
| (95% CI) | (-29.2%, -16.0%) | (-13.1%, -0.5%) | ||
| Non-Inflammatory Lesions | ||||
| Mean† Absolute Change from Baseline | -29.7 | -19.8 | -24.2 | -17.4 |
| Difference from Vehicle | -9.9 | -6.8 | ||
| (95% CI) | (-13.0, -6.8) | (-9.9, -3.7) | ||
| Mean† Percent Change from Baseline | -61.6% | -40.9% | -54.4% | -41.5% |
| Difference from Vehicle | -20.7% | -13.0% | ||
| (95% CI) | (-27.2%, -14.2%) | (-19.6%, -6.4%) | ||
16. How is Twyneo supplied
How Supplied
TWYNEO (tretinoin and benzoyl peroxide) cream, 0.1%/3% is a yellow cream and is supplied as:
- 30-gram bottle with a pump, NDC 51862-771-30
Storage and Handling
- Prior to Dispensing: Store TWYNEO between 2°C to 8°C (36°F to 46°F) until dispensed to the patient.
- After Dispensing: Store TWYNEO at room temperature between 20°C to 25°C (68°F to 77°F). Discard 12 weeks after date of dispensing or 60 days after first opening, whichever is sooner.
- Do not freeze.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity
Inform patients that serious hypersensitivity reactions have occurred with the use of benzoyl peroxide products. If a patient experiences a serious hypersensitivity reaction, instruct patient to discontinue TWYNEO immediately and seek medical help [see Warnings and Precautions (5.1)].
Skin Irritation
Inform patients that TWYNEO may cause irritation such as erythema, dryness, stinging or burning. Advise the patient to use a moisturizer for irritation. [see Warnings and Precautions (5.2)].
Photosensitivity
Advise patients to minimize unprotected exposure to sunlight and sunlamps; recommend the use of sunscreen products and protective apparel (e.g., hat) over treated areas when sun exposure cannot be avoided. [see Warnings and Precautions (5.3)].
Administration Instructions
Advise patients to apply TWYNEO exactly as directed in a thin layer, avoiding the eyes, lips, paranasal creases and mucous membranes and to wash hands immediately after application. Inform patients that TWYNEO may bleach hair or colored fabric [see Dosage and Administration (2)].
Discard Instructions
Instruct patients to store TWYNEO at room temperature and to discard 12 weeks after date of dispensing or 60 days after first opening, whichever is sooner. [see How Supplied/Storage and Handling (16)].
Distributed by:
Mayne Pharma
Raleigh, NC 27609
Made in New Zealand
All trademarks are the property of their respective owners
PATIENT INFORMATION
TWYNEO ® (Twye'nee oh)
(tretinoin and benzoyl peroxide)
cream, for topical use
Important: TWYNEO is for use on the skin only (topical). Do not use TWYNEO in your mouth, eyes, or vagina.
What is TWYNEO?
TWYNEO is a prescription medicine used on the skin (topical) to treat acne vulgaris in adults and children 9 years of age and older.
It is not known if TWYNEO is safe and effective in children below 9 years of age.
Do not use TWYNEO if you have had an allergic reaction to benzoyl peroxide or any of the ingredients in TWYNEO. See the end of this leaflet for a complete list of ingredients in TWYNEO.
Before using TWYNEO, tell your healthcare provider about all of your medical conditions, including if you:
- have other skin problems, including eczema, cuts, or sunburn
- have skin sensitivity to the sun
- are pregnant or planning to become pregnant. It is not known if TWYNEO wi l harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if TWYNEO passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with TWYNEO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use TWYNEO?
- Use TWYNEO exactly as your healthcare provider tells you to use it.
- Apply TWYNEO to the affected areas 1 time a day.
- Apply TWYNEO on clean and dry skin.
- TWYNEO come in a bottle with a pump. Press down on (depress) the pump to dispense a small amount of TWYNEO on your fingertip. Apply a thin layer of TWYNEO to the affected areas. Avoid contact with your eyes, lips, corners of your nose, and mouth.
- Wash your hands right away after applying TWYNEO.
What should I avoid while using TWYNEO?
- Avoid using TWYNEO on skin areas with cuts, abrasions, eczema, or sunburns.
- Limit your time in sunlight. You should avoid using sunlamps, tanning beds, and ultraviolet light during treatment with TWYNEO. If you have to be in the sunlight or are sensitive to sunlight, use sunscreen and wear protective clothing or a wide- brimmed hat to cover the treated areas.
- Avoid getting TWYNEO in your hair or on colored fabric. TWYNEO may bleach hair or colored fabric.
What are the possible side effects of TWYNEO? TWYNEO may cause serious side effects including:
-
Allergic reactions. Stop using TWYNEO and get medical help right away if you have any of the following symptoms during treatment with TWYNEO:
- hives, rash or severe itching
- swelling of your face, eyes, lips, tongue, or throat
- trouble breathing or throat tightness
- feeling faint, dizzy, or lightheaded
-
Skin irritation. TWYNEO may cause skin irritation such as redness, scaling, peeling, dryness, pain, stinging or burning. If you develop these symptoms, your healthcare provider may tell you to use a moisturizer, decrease the number of times you
apply TWYNEO, or completely stop treatment of TWYNEO.
The most common side effects of TWYNEO include pain, dryness, peeling, redness, swe ling, itching, and irritation at the application site.
These are not all possible side effects of TWYNEO.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
You may also report side effects to Galderma Laboratories, L.P. at 1-866-735-4137.
How should I store TWYNEO?
- Store TWYNEO at room temperature between 68°F to 77°F (20C° to 25°C).
- Throw away (discard) TWYNEO 12 weeks after the date you receive it or 60 days after first opening, whichever is sooner.
- Do not freeze.
Keep TWYNEO and all medicines out of reach of children.
General information about the safe and effective use of TWYNEO. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use TWYNEO for a condition for which it was not prescribed. Do not use TWYNEO for a condition for which it was not prescribed. Do not give TWYNEO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about TWYNEO that is written for health professionals.
What are the ingredients in TWYNEO?
Active Ingredients: tretinoin and benzoyl peroxide
Inactive Ingredients: anhydrous citric acid, butylated hydroxytoluene, carbomer homopolymer type C, cetrimonium chloride, cetyl alcohol, cyclomethicone, edetate disodium, glycerin, hydrochloric acid, imidurea, (S)-lactic acid, macrogol stearate, mono and di-glycerides, polyquaternium-7, purified water, silicon dioxide, sodium hydroxide, squalane, tetraethyl ortho silicate and white wax.
| TWYNEO
benzoyl peroxide and tretinoin cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Mayne Pharma Commercial LLC (867220261) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Douglas Manufacturing Limited | 590829388 | MANUFACTURE(51862-771) , ANALYSIS(51862-771) , PACK(51862-771) , LABEL(51862-771) | |
Frequently asked questions
More about Twyneo (benzoyl peroxide / tretinoin topical)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: topical acne agents
- En español