Trilipix: Package Insert / Prescribing Info
Package insert / product label
Generic name: fenofibric acid
Dosage form: capsule, delayed release
Drug class: Fibric acid derivatives
Medically reviewed by Drugs.com. Last updated on Dec 2, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
TRILIPIX (fenofibric acid) capsule, delayed release for oral use
Initial U.S. Approval: 2008
Indications and Usage for Trilipix
Trilipix is a peroxisome proliferator-activated receptor (PPAR) alpha agonist indicated as adjunctive therapy to diet to:
- Reduce TG in patients with severe hypertriglyceridemia (1.1).
- Reduce elevated LDL-C, Total-C, TG and Apo B, and to increase HDL-C in patients with primary hypercholesterolemia or mixed dyslipidemia (1.2).
Limitations of Use: Fenofibrate at a dose equivalent to 135 mg of Trilipix did not reduce coronary heart disease morbidity and mortality in patients with type 2 diabetes mellitus (5.1).
Trilipix Dosage and Administration
Dosage Forms and Strengths
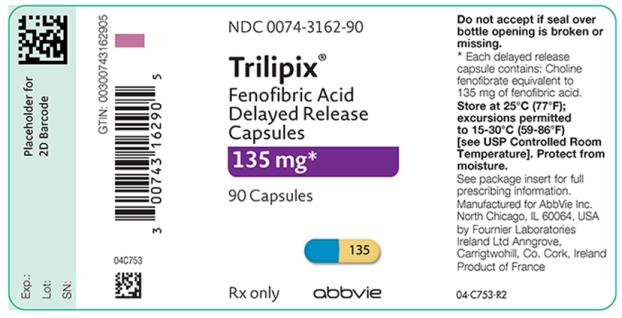
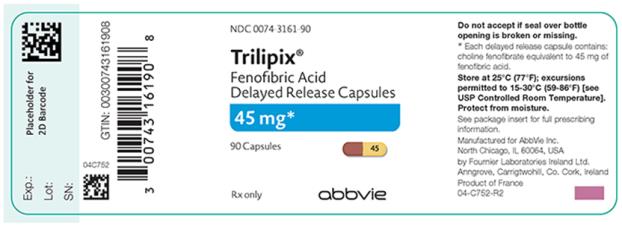
Oral Delayed Release Capsules: 45 mg and 135 mg (3).
Contraindications
Warnings and Precautions
-
Hepatotoxicity: Serious drug-induced liver injury, including liver transplantation and death, has been reported with Trilipix. Monitor patient’s liver function, including serum ALT, AST, and total bilirubin, at baseline and periodically for the duration of therapy. Discontinue if signs or symptoms of liver injury develop or if elevated enzyme levels persist (5.2).
-
Myopathy and rhabdomyolysis: Have been reported in patients taking fenofibrate. Risks are increased in elderly patients and patients with diabetes, renal failure, hypothyroidism, or statin co-administration (5.3).
-
Serum creatinine: Trilipix can reversibly increase serum creatinine levels (5.4). Monitor renal function periodically in patients with renal insufficiency (8.6).
-
Cholelithiasis: Trilipix increases cholesterol excretion into the bile, leading to risk of cholelithiasis. If suspected, gallbladder studies are indicated (5.5).
-
Coumarin anticoagulants: Use caution in concomitant treatment with oral coumarin anticoagulants. Adjust the dosage of coumarin anticoagulant to maintain the prothrombin time/INR at the desired level to prevent bleeding complications (5.6).
- Hypersensitivity reactions: Acute hypersensitivity reactions, including anaphylaxis and angioedema, and delayed hypersensitivity reactions, including severe cutaneous adverse drug reactions have been reported postmarketing. Some cases were life-threatening and required emergency treatment. Discontinue fenofibrate and treat patients appropriately if reactions occur (5.9).
Adverse Reactions/Side Effects
The most common adverse events reported during clinical trials with fenofibrate (≥ 2% and at least 1% greater than placebo) were abnormal liver tests, increased AST, increased ALT, increased CPK, and rhinitis (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact AbbVie Inc. at 1-800-633-9110 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Use In Specific Populations
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2024
Full Prescribing Information
1. Indications and Usage for Trilipix
1.1 Treatment of Severe Hypertriglyceridemia
Trilipix is indicated as adjunctive therapy to diet to reduce triglycerides (TG) in patients with severe hypertriglyceridemia. Improving glycemic control in diabetic patients showing fasting chylomicronemia will usually obviate the need for pharmacological intervention. Markedly elevated levels of serum triglycerides (e.g. > 2,000 mg/dL) may increase the risk of developing pancreatitis. The effect of Trilipix therapy on reducing this risk has not been adequately studied.
1.2 Treatment of Primary Hypercholesterolemia or Mixed Dyslipidemia
Trilipix is indicated as adjunctive therapy to diet to reduce elevated low-density lipoprotein cholesterol (LDL-C), total cholesterol (Total-C), triglycerides (TG), and apolipoprotein B (Apo B), and to increase high-density lipoprotein cholesterol (HDL-C) in patients with primary hypercholesterolemia or mixed dyslipidemia.
1.3 Limitations of Use
Fenofibrate at a dose equivalent to 135 mg of Trilipix did not reduce coronary heart disease morbidity and mortality in 2 large, randomized controlled trials of patients with type 2 diabetes mellitus [see Warnings and Precautions (5.1)].
1.4 General Considerations for Treatment
Laboratory studies should be performed to establish that lipid levels are abnormal before instituting Trilipix therapy.
Every reasonable attempt should be made to control serum lipids with non-drug methods including appropriate diet, exercise, weight loss in obese patients, and control of any medical problems such as diabetes mellitus and hypothyroidism that may be contributing to the lipid abnormalities. Medications known to exacerbate hypertriglyceridemia (beta-blockers, thiazides, estrogens) should be discontinued or changed if possible, and excessive alcohol intake should be addressed before triglyceride-lowering drug therapy is considered. If the decision is made to use lipid-altering drugs, the patient should be instructed that this does not reduce the importance of adhering to diet.
Drug therapy is not indicated for patients who have elevations of chylomicrons and plasma triglycerides, but who have normal levels of VLDL.
2. Trilipix Dosage and Administration
2.1 General Considerations
Patients should be placed on an appropriate lipid-lowering diet before receiving Trilipix and should continue this diet during treatment. Trilipix delayed release capsules can be taken without regard to meals. Patients should be advised to swallow Trilipix capsules whole. Do not open, crush, dissolve, or chew capsules. Serum lipids should be monitored periodically.
2.2 Severe Hypertriglyceridemia
The initial dose of Trilipix is 45 to 135 mg once daily. Dosage should be individualized according to patient response, and should be adjusted if necessary following repeat lipid determinations at 4 to 8 week intervals. The maximum dose is 135 mg once daily.
2.4 Impaired Renal Function
Treatment with Trilipix should be initiated at a dose of 45 mg once daily in patients with mild to moderate renal impairment and should only be increased after evaluation of the effects on renal function and lipid levels at this dose. The use of Trilipix should be avoided in patients with severely impaired renal function [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
2.5 Geriatric Patients
Dose selection for the elderly should be made on the basis of renal function [see Use in Specific Populations (8.5)].
3. Dosage Forms and Strengths
- 45 mg capsules with a reddish brown to orange brown cap and a yellow body imprinted in black ink the number “45”.
- 135 mg capsules with a blue cap and a yellow body imprinted in black ink the number “135”.
4. Contraindications
Trilipix is contraindicated in:
- patients with severe renal impairment, including those receiving dialysis [see Clinical Pharmacology (12.3)].
- patients with active liver disease, including those with primary biliary cirrhosis and unexplained persistent liver function abnormalities [see Warnings and Precautions (5.2)].
- patients with preexisting gallbladder disease [see Warnings and Precautions (5.5)].
- nursing mothers [see Use in Specific Populations (8.2)].
- patients with hypersensitivity to fenofibric acid or fenofibrate [see Warnings and Precautions (5.9)].
5. Warnings and Precautions
5.1 Mortality and Coronary Heart Disease Morbidity
The effect of Trilipix on coronary heart disease morbidity and mortality and non-cardiovascular mortality has not been established. Because of similarities between Trilipix and fenofibrate, clofibrate, and gemfibrozil, the findings in the following large randomized, placebo-controlled clinical studies with these fibrate drugs may also apply to Trilipix.
The Action to Control Cardiovascular Risk in Diabetes Lipid (ACCORD Lipid) trial was a randomized placebo-controlled study of 5518 patients with type 2 diabetes mellitus on background statin therapy treated with fenofibrate. The mean duration of follow-up was 4.7 years. Fenofibrate plus statin combination therapy showed a non-significant 8% relative risk reduction in the primary outcome of major adverse cardiovascular events (MACE), a composite of non-fatal myocardial infarction, non-fatal stroke, and cardiovascular disease death (hazard ratio [HR] 0.92, 95% CI 0.79-1.08) (p=0.32) as compared to statin monotherapy. In a gender subgroup analysis, the hazard ratio for MACE in men receiving combination therapy versus statin monotherapy was 0.82 (95% CI 0.69-0.99), and the hazard ratio for MACE in women receiving combination therapy versus statin monotherapy was 1.38 (95% CI 0.98-1.94) (interaction p=0.01). The clinical significance of this subgroup finding is unclear.
The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study was a 5-year randomized, placebo-controlled study of 9795 patients with type 2 diabetes mellitus treated with fenofibrate. Fenofibrate demonstrated a non-significant 11% relative reduction in the primary outcome of coronary heart disease events (hazard ratio [HR] 0.89, 95% CI 0.75-1.05, p = 0.16) and a significant 11% reduction in the secondary outcome of total cardiovascular disease events (HR 0.89 [0.80-0.99], p = 0.04). There was a non-significant 11% (HR 1.11 [0.95, 1.29], p = 0.18) and 19% (HR 1.19 [0.90, 1.57], p = 0.22) increase in total and coronary heart disease mortality, respectively, with fenofibrate as compared to placebo.
In the Coronary Drug Project, a large study of post-myocardial infarction patients treated for 5 years with clofibrate, there was no difference in mortality seen between the clofibrate group and the placebo group. There was, however, a difference in the rate of cholelithiasis and cholecystitis requiring surgery between the two groups (3.0% vs. 1.8%).
In a study conducted by the World Health Organization (WHO), 5000 subjects without known coronary artery disease were treated with placebo or clofibrate for 5 years and followed for an additional one year. There was a statistically significant, higher age-adjusted all-cause mortality in the clofibrate group compared with the placebo group (5.70% vs. 3.96%, p = < 0.01). Excess mortality was due to a 33% increase in non-cardiovascular causes, including malignancy, post-cholecystectomy complications, and pancreatitis. This appeared to confirm the higher risk of gallbladder disease seen in clofibrate-treated patients studied in the Coronary Drug Project.
The Helsinki Heart Study was a large (N = 4081) study of middle-aged men without a history of coronary artery disease. Subjects received either placebo or gemfibrozil for 5 years, with a 3.5 year open extension afterward. Total mortality was numerically higher in the gemfibrozil randomization group but did not achieve statistical significance (p = 0.19, 95% confidence interval for relative risk G:P = 0.91-1.64). Although cancer deaths trended higher in the gemfibrozil group (p = 0.11), cancers (excluding basal cell carcinoma) were diagnosed with equal frequency in both study groups. Due to the limited size of the study, the relative risk of death from any cause was not shown to be different than that seen in the 9 year follow-up data from WHO study (RR = 1.29). A secondary prevention component of the Helsinki Heart Study enrolled middle-aged men excluded from the primary prevention study because of known or suspected coronary heart disease. Subjects received gemfibrozil or placebo for 5 years. Although cardiac deaths trended higher in the gemfibrozil group, this was not statistically significant (hazard ratio 2.2, 95% confidence interval: 0.94-5.05).
5.2 Hepatotoxicity
Serious drug-induced liver injury (DILI), including liver transplantation and death, have been reported postmarketing with Trilipix. DILI has been reported within the first few weeks of treatment or after several months of therapy and in some cases has reversed with discontinuation of Trilipix treatment. Patients with DILI have experienced signs and symptoms including dark urine, abnormal stool, jaundice, malaise, abdominal pain, myalgia, weight loss, pruritus, and nausea. Many patients had concurrent elevations of total bilirubin, serum alanine transaminase (ALT), and aspartate transaminase (AST). DILI has been characterized as hepatocellular, chronic active, and cholestatic hepatitis, and cirrhosis has occurred in association with chronic active hepatitis.
In clinical trials, Trilipix at a dose of 135 mg daily has been associated with increases in serum AST or ALT. The incidence of increases in transaminases observed with fenofibrate therapy may be dose related [see Adverse Reactions (6.1)].
Trilipix is contraindicated in patients with active liver disease, including those with primary biliary cirrhosis and unexplained persistent liver function abnormalities [see Contraindications (4)]. Monitor patient’s liver function, including serum ALT, AST, and total bilirubin, at baseline and periodically for the duration of therapy with Trilipix. Discontinue Trilipix if signs or symptoms of liver injury develop or if elevated enzyme levels persist (ALT or AST > 3 times the upper limit of normal, or if accompanied by elevation of bilirubin). Do not restart Trilipix in these patients if there is no alternative explanation for the liver injury.
5.3 Myopathy and Rhabdomyolysis
Fibrates increase the risk of myositis or myopathy and have been associated with rhabdomyolysis. The risk for serious muscle toxicity appears to be increased in elderly patients and in patients with diabetes, renal failure, or hypothyroidism.
Myopathy should be considered in any patient with diffuse myalgias, muscle tenderness or weakness, and/or marked elevations of CPK levels. Patients should promptly report unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever. CPK levels should be assessed in patients reporting these symptoms, and Trilipix should be discontinued if markedly elevated CPK levels occur or myopathy or myositis is suspected or diagnosed.
Data from observational studies suggest that the risk for rhabdomyolysis is increased when fibrates are co-administered with a statin.
Cases of myopathy, including rhabdomyolysis, have been reported with fenofibrates co-administered with colchicine, and caution should be exercised when prescribing fenofibrate with colchicine [see Drug Interactions (7.4)].
5.4 Serum Creatinine
Reversible elevations in serum creatinine have been reported in patients receiving Trilipix as well as patients receiving fenofibrate. In the pooled analysis of three 12-week, double-blind, controlled studies of Trilipix, increases in creatinine to > 2 mg/dL occurred in 0.8% of patients treated with Trilipix without other lipid-altering drugs. Elevations in serum creatinine were generally stable over time with no evidence for continued increases in serum creatinine with long-term therapy and tended to return to baseline following discontinuation of treatment. The clinical significance of these observations is unknown. Monitoring renal function in patients with renal impairment taking Trilipix is suggested. Renal monitoring should be considered for patients at risk for renal insufficiency, such as the elderly and those with diabetes.
5.5 Cholelithiasis
Trilipix, like fenofibrate, clofibrate, and gemfibrozil, may increase cholesterol excretion into the bile, potentially leading to cholelithiasis. If cholelithiasis is suspected, gallbladder studies are indicated. Trilipix therapy should be discontinued if gallstones are found.
5.6 Coumarin Anticoagulants
Caution should be exercised when Trilipix is given in conjunction with oral coumarin anticoagulants. Trilipix may potentiate the anticoagulant effects of these agents resulting in prolongation of the prothrombin time/International Normalized Ratio (PT/INR). Frequent monitoring of PT/INR and dose adjustment of the oral anticoagulant are recommended until the PT/INR has stabilized in order to prevent bleeding complications [see Drug Interactions (7.1)].
5.7 Pancreatitis
Pancreatitis has been reported in patients taking drugs of the fibrate class, including Trilipix. This occurrence may represent a failure of efficacy in patients with severe hypertriglyceridemia, a direct drug effect, or a secondary phenomenon mediated through biliary tract stone or sludge formation with obstruction of the common bile duct.
5.8 Hematological Changes
Mild to moderate hemoglobin, hematocrit, and white blood cell decreases have been observed in patients following initiation of Trilipix and fenofibrate therapy. However, these levels stabilize during long-term administration. Thrombocytopenia and agranulocytosis have been reported in individuals treated with fenofibrates. Periodic monitoring of red and white blood cell counts are recommended during the first 12 months of Trilipix administration.
5.9 Hypersensitivity Reactions
Acute Hypersensitivity
Anaphylaxis and angioedema have been reported postmarketing with fenofibrate. In some cases, reactions were life-threatening and required emergency treatment. If a patient develops signs or symptoms of an acute hypersensitivity reaction, advise them to seek immediate medical attention and discontinue fenofibrate.
Delayed Hypersensitivity
Severe cutaneous adverse drug reactions (SCAR), including Stevens-Johnson syndrome, toxic epidermal necrolysis, and Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), have been reported postmarketing, occurring days to weeks after initiation of fenofibrate. The cases of DRESS were associated with cutaneous reactions (such as rash or exfoliative dermatitis) and a combination of eosinophilia, fever, systemic organ involvement (renal, hepatic, or respiratory). Discontinue fenofibrate and treat patients appropriately if SCAR is suspected.
5.10 Venothromboembolic Disease
In the FIELD trial, pulmonary embolus (PE) and deep vein thrombosis (DVT) were observed at higher rates in the fenofibrate- than the placebo-treated group. Of 9,795 patients enrolled in FIELD, there were 4,900 in the placebo group and 4,895 in the fenofibrate group. For DVT, there were 48 events (1%) in the placebo group and 67 (1%) in the fenofibrate group (p = 0.074); and for PE, there were 32 (0.7%) events in the placebo group and 53 (1%) in the fenofibrate group (p = 0.022).
In the Coronary Drug Project, a higher proportion of the clofibrate group experienced definite or suspected fatal or nonfatal PE or thrombophlebitis than the placebo group (5.2% vs. 3.3% at five years; p < 0.01).
5.11 Paradoxical Decreases in HDL Cholesterol Levels
There have been postmarketing and clinical trial reports of severe decreases in HDL cholesterol levels (as low as 2 mg/dL) occurring in diabetic and non-diabetic patients initiated on fibrate therapy. The decrease in HDL-C is mirrored by a decrease in apolipoprotein A1. This decrease has been reported to occur within 2 weeks to years after initiation of fibrate therapy. The HDL-C levels remain depressed until fibrate therapy has been withdrawn; the response to withdrawal of fibrate therapy is rapid and sustained. The clinical significance of this decrease in HDL-C is unknown. It is recommended that HDL-C levels be checked within the first few months after initiation of fibrate therapy. If a severely depressed HDL-C level is detected, fibrate therapy should be withdrawn, and the HDL-C level monitored until it has returned to baseline, and fibrate therapy should not be re-initiated.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described below and elsewhere in the labeling:
- Mortality and coronary heart disease morbidity [see Warnings and Precautions (5.1)]
- Hepatoxicity [see Warnings and Precautions (5.2)]
- Pancreatitis [see Warnings and Precautions (5.7)]
- Hypersensitivity reactions [see Warnings and Precautions (5.9)]
- Venothromboembolic disease [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Fenofibric acid is the active metabolite of fenofibrate. Adverse events reported by 2% or more of patients treated with fenofibrate and greater than placebo during double-blind, placebo-controlled trials are listed in Table 1. Adverse events led to discontinuation of treatment in 5.0% of patients treated with fenofibrate and in 3.0% treated with placebo. Increases in liver tests were the most frequent events, causing discontinuation of fenofibrate treatment in 1.6% of patients in double-blind trials.
| BODY SYSTEM
Adverse Event | Fenofibrate*
(N = 439) | Placebo
(N = 365) |
| BODY AS A WHOLE | ||
| Abdominal Pain | 4.6% | 4.4% |
| Back Pain | 3.4% | 2.5% |
| Headache | 3.2% | 2.7% |
| DIGESTIVE | ||
| Nausea | 2.3% | 1.9% |
| Constipation | 2.1% | 1.4% |
| INVESTIGATIONS | ||
| Abnormal Liver Tests | 7.5% | 1.4% |
| Increased AST | 3.4% | 0.5% |
| Increased ALT | 3.0% | 1.6% |
| Increased Creatine Phosphokinase | 3.0% | 1.4% |
| RESPIRATORY | ||
| Respiratory Disorder | 6.2% | 5.5% |
| Rhinitis | 2.3% | 1.1% |
| * Dosage equivalent to 135 mg Trilipix | ||
Urticaria was seen in 1.1% vs. 0%, and rash in 1.4% vs. 0.8% of fenofibrate and placebo patients respectively in controlled trials.
Clinical trials with Trilipix did not include a placebo-control arm. However, the adverse event profile of Trilipix was generally consistent with that of fenofibrate. The following adverse events not listed above were reported in ≥ 3% of patients taking Trilipix alone:
Gastrointestinal Disorders: Diarrhea, dyspepsia
General Disorders and Administration Site Conditions: Pain
Infections and Infestations: Nasopharyngitis, sinusitis, upper respiratory tract infection
Musculoskeletal and Connective Tissue Disorders: Arthralgia, myalgia, pain in extremity
Nervous System Disorders: Dizziness
Increases in Liver Enzymes
In a pooled analysis of three 12-week, double-blind, controlled studies of Trilipix, increases in ALT and AST > 3 times the upper limit of normal on two consecutive occasions occurred in 1.9% and 0.2%, respectively, of patients receiving Trilipix 135 mg daily and placebo, without other lipid-altering drugs. In a pooled analysis of 10 placebo-controlled trials, increases to > 3 times the upper limit of normal in ALT occurred in 5.3% of patients taking fenofibrate versus 1.1% of patients treated with placebo. In an 8-week study, the incidence of ALT or AST elevations ≥ 3 times the upper limit of normal was 13% in patients receiving dosages equivalent to 90 mg to 135 mg Trilipix daily and was 0% in those receiving dosages equivalent to 45 mg or less Trilipix daily or placebo.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of fenofibrate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: rhabdomyolysis, pancreatitis, renal failure, muscle spasms, acute renal failure, hepatitis, cirrhosis, increased total bilirubin, anemia, asthenia, severely depressed HDL-cholesterol levels, and interstitial lung disease. Photosensitivity reactions to fenofibrate have occurred days to months after initiation; in some of these cases, patients reported a prior photosensitivity reaction to ketoprofen.
Related/similar drugs
7. Drug Interactions
7.1 Coumarin Anticoagulants
Potentiation of coumarin-type anticoagulant effect has been observed with prolongation of the PT/INR.
Caution should be exercised when oral coumarin anticoagulants are given in conjunction with Trilipix. The dosage of the anticoagulant should be reduced to maintain the PT/INR at the desired level to prevent bleeding complications. Frequent PT/INR determinations are advisable until it has been definitely determined that the PT/INR has stabilized [see Warnings and Precautions (5.6)].
7.2 Bile Acid Binding Resins
Since bile acid binding resins may bind other drugs given concurrently, patients should take Trilipix at least 1 hour before or 4 to 6 hours after a bile acid resin to avoid impeding its absorption.
7.3 Immunosuppressants
Immunosuppressants such as cyclosporine and tacrolimus can produce nephrotoxicity with decreases in creatinine clearance and rises in serum creatinine, and because renal excretion is the primary elimination route of drugs of the fibrate class including Trilipix, there is a risk that an interaction will lead to deterioration of renal function. The benefits and risks of using Trilipix with immunosuppressants and other potentially nephrotoxic agents should be carefully considered, and the lowest effective dose employed.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Limited available data with fenofibrate use in pregnant women are insufficient to determine a drug associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. In animal reproduction studies, no evidence of embryo-fetal toxicity was observed with oral administration of fenofibrate in rats and rabbits during organogenesis at doses less than or equivalent to the maximum recommended clinical dose of 135 mg daily, based on body surface area (mg/m2). Adverse reproductive outcomes occurred at higher doses in the presence of maternal toxicity (see Data). Trilipix should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In pregnant rats given oral dietary doses of 14, 127, and 361 mg/kg/day from gestation day 6-15 during the period of organogenesis, no adverse developmental findings were observed at 14 mg/kg/day (less than the clinical exposure at the maximum recommended human dose [MRHD] of 300 mg fenofibrate daily, equivalent to 135 mg Trilipix daily, based on body surface area comparisons). Increased fetal skeletal malformations were observed at maternally toxic doses (361 mg/kg/day, corresponding to 12 times the clinical exposure at the MRHD) that significantly suppressed maternal body weight gain.
In pregnant rabbits given oral gavage doses of 15, 150, and 300 mg/kg/day from gestation day 6-18 during the period of organogenesis and allowed to deliver, no adverse developmental findings were observed at 15 mg/kg/day (a dose that approximates the clinical exposure at the MRHD, based on body surface area comparisons). Aborted litters were observed at maternally toxic doses (≥ 150 mg/kg/day, corresponding to ≥ 10 times the clinical exposure at the MRHD) that suppressed maternal body weight gain.
In pregnant rats given oral dietary doses of 15, 75, and 300 mg/kg/day from gestation day 15 through lactation day 21 (weaning), no adverse developmental effects were observed at 15 mg/kg/day (less than the clinical exposure at the MRHD, based on body surface area comparisons), despite maternal toxicity (decreased weight gain). Post-implantation loss was observed at ≥ 75 mg/kg/day (≥ 2 times the clinical exposure at the MRHD) in the presence of maternal toxicity (decreased weight gain). Decreased pup survival was noted at 300 mg/kg/day (10 times the clinical exposure at the MRHD), which was associated with decreased maternal body weight gain/maternal neglect.
8.2 Lactation
Risk Summary
There is no available information on the presence of fenofibrate in human milk, effects of the drug on the breastfed infant, or the effects on milk production. Fenofibrate is present in the milk of rats, and is therefore likely to be present in human milk. Because of the potential for serious adverse reactions in breastfed infants, such as disruption of infant lipid metabolism, women should not breastfeed during treatment with Trilipix and for 5 days after the final dose [see Contraindications (4)].
8.4 Pediatric Use
The safety and effectiveness of Trilipix in pediatric patients have not been established.
8.5 Geriatric Use
Trilipix is substantially excreted by the kidney as fenofibric acid and fenofibric acid glucuronide, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Fenofibric acid exposure is not influenced by age. Since elderly patients have a higher incidence of renal impairment, dose selection for the elderly should be made on the basis of renal function [see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)]. Elderly patients with normal renal function should require no dose modifications. Consider monitoring renal function in elderly patients taking Trilipix.
8.6 Renal Impairment
The use of Trilipix should be avoided in patients who have severe renal impairment [see Contraindications (4)]. Dose reduction is required in patients with mild to moderate renal impairment [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)]. Monitoring renal function in patients with renal impairment is recommended.
10. Overdosage
There is no specific treatment for overdose with Trilipix. General supportive care of the patient is indicated, including monitoring of vital signs and observation of clinical status, should an overdose occur. If indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage; usual precautions should be observed to maintain the airway. Because Trilipix is highly bound to plasma proteins, hemodialysis should not be considered.
11. Trilipix Description
Trilipix (fenofibric acid) is a lipid regulating agent available as delayed release capsules for oral administration. Each delayed release capsule contains choline fenofibrate, equivalent to 45 mg or 135 mg of fenofibric acid. The chemical name for choline fenofibrate is ethanaminium, 2-hydroxy-N,N,N-trimethyl, 2-{4-(4-chlorobenzoyl)phenoxy] -2-methylpropanoate (1:1) with the following structural formula:
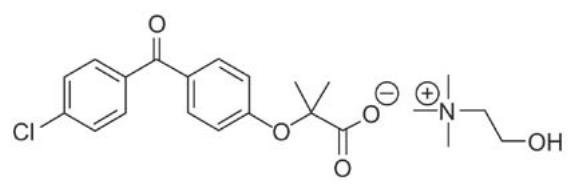
The empirical formula is C22H28ClNO5 and the molecular weight is 421.91. Choline fenofibrate is freely soluble in water. The melting point is approximately 210°C. Choline fenofibrate is a white to yellow powder, which is stable under ordinary conditions.
Each delayed release capsule contains enteric coated mini-tablets comprised of choline fenofibrate and the following inactive ingredients: hypromellose, povidone, water, hydroxypropyl cellulose, colloidal silicon dioxide, sodium stearyl fumarate, methacrylic acid copolymer, talc, triethyl citrate. The capsule shell of the 45 mg capsule contains the following inactive ingredients: gelatin, titanium dioxide, yellow iron oxide, black iron oxide, and red iron oxide. The capsule shell of the 135 mg capsule contains the following inactive ingredients: gelatin, titanium dioxide, yellow iron oxide, and FD&C Blue #2.
12. Trilipix - Clinical Pharmacology
12.1 Mechanism of Action
The active moiety of Trilipix is fenofibric acid. The pharmacological effects of fenofibric acid in both animals and humans have been extensively studied through oral administration of fenofibrate.
The lipid-modifying effects of fenofibric acid seen in clinical practice have been explained in vivo in transgenic mice and in vitro in human hepatocyte cultures by the activation of peroxisome proliferator activated receptor α (PPARα). Through this mechanism, fenofibric acid increases lipolysis and elimination of triglyceride-rich particles from plasma by activating lipoprotein lipase and reducing production of Apo CIII (an inhibitor of lipoprotein lipase activity).
Activation of PPARα also induces an increase in the synthesis of HDL-C and Apo AI and AII.
12.3 Pharmacokinetics
Trilipix contains fenofibric acid, which is the only circulating pharmacologically active moiety in plasma after oral administration of Trilipix. Fenofibric acid is also the circulating pharmacologically active moiety in plasma after oral administration of fenofibrate, the ester of fenofibric acid.
Plasma concentrations of fenofibric acid after administration of one 135 mg Trilipix delayed release capsule are equivalent to those after one 200 mg capsule of micronized fenofibrate administered under fed conditions.
Absorption
Fenofibric acid is well absorbed throughout the gastrointestinal tract. The absolute bioavailability of fenofibric acid is approximately 81%.
Peak plasma levels of fenofibric acid occur within 4 to 5 hours after a single dose administration of Trilipix capsule under fasting conditions.
Fenofibric acid exposure in plasma, as measured by Cmax and AUC, is not significantly different when a single 135 mg dose of Trilipix is administered under fasting or nonfasting conditions.
Distribution
Upon multiple dosing of Trilipix, fenofibric acid levels reach steady state within 8 days. Plasma concentrations of fenofibric acid at steady state are approximately slightly more than double those following a single dose. Serum protein binding is approximately 99% in normal and dyslipidemic subjects.
Metabolism
Fenofibric acid is primarily conjugated with glucuronic acid and then excreted in urine. A small amount of fenofibric acid is reduced at the carbonyl moiety to a benzhydrol metabolite which is, in turn, conjugated with glucuronic acid and excreted in urine.
In vivo metabolism data after fenofibrate administration indicate that fenofibric acid does not undergo oxidative metabolism (e.g., cytochrome P450) to a significant extent.
Elimination
After absorption, Trilipix is primarily excreted in the urine in the form of fenofibric acid and fenofibric acid glucuronide.
Fenofibric acid is eliminated with a half-life of approximately 20 hours, allowing once daily administration of Trilipix.
Specific Populations
Geriatrics
In five elderly volunteers 77 to 87 years of age, the oral clearance of fenofibric acid following a single oral dose of fenofibrate was 1.2 L/h, which compares to 1.1 L/h in young adults. This indicates that an equivalent dose of Trilipix can be used in elderly subjects with normal renal function, without increasing accumulation of the drug or metabolites [see Use in Specific Populations (8.5)].
Pediatrics
The pharmacokinetics of Trilipix has not been studied in pediatric populations.
Gender
No pharmacokinetic difference between males and females has been observed for Trilipix.
Race
The influence of race on the pharmacokinetics of Trilipix has not been studied; however, fenofibric acid is not metabolized by enzymes known for exhibiting inter-ethnic variability.
Renal Impairment
The pharmacokinetics of fenofibric acid was examined in patients with mild, moderate, and severe renal impairment. Patients with severe renal impairment (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73m2) showed a 2.7-fold increase in exposure for fenofibric acid and increased accumulation of fenofibric acid during chronic dosing compared to that of healthy subjects. Patients with mild to moderate renal impairment (eGFR 30-59 mL/min/1.73m2) had similar exposure but an increase in the half-life for fenofibric acid compared to that of healthy subjects. Based on these findings, the use of Trilipix should be avoided in patients who have severe renal impairment and dose reduction is required in patients having mild to moderate renal impairment [see Dosage and Administration (2.4)].
Hepatic Impairment
No pharmacokinetic studies have been conducted in patients with hepatic impairment.
Drug-drug Interactions
In vitro studies using human liver microsomes indicate that fenofibric acid is not an inhibitor of cytochrome (CYP) P450 isoforms CYP3A4, CYP2D6, CYP2E1, or CYP1A2. It is a weak inhibitor of CYP2C8, CYP2C19, and CYP2A6, and mild-to-moderate inhibitor of CYP2C9 at therapeutic concentrations.
Comparison of atorvastatin exposures when atorvastatin (80 mg once daily for 10 days) is given in combination with fenofibric acid (Trilipix 135 mg once daily for 10 days) and ezetimibe (10 mg once daily for 10 days) versus when atorvastatin is given in combination with ezetimibe only (ezetimibe 10 mg once daily and atorvastatin, 80 mg once daily for 10 days): The Cmax decreased by 1% for atorvastatin and ortho-hydroxy-atorvastatin and increased by 2% for parahydroxy-atorvastatin. The AUC decreased 6% and 9% for atorvastatin and orthohydroxy-atorvastatin, respectively, and did not change for para-hydroxy-atorvastatin.
Comparison of ezetimibe exposures when ezetimibe (10 mg once daily for 10 days) is given in combination with fenofibric acid (Trilipix 135 mg once daily for 10 days) and atorvastatin (80 mg once daily for 10 days) versus when ezetimibe is given in combination with atorvastatin only (ezetimibe 10 mg once daily and atorvastatin, 80 mg once daily for 10 days): The Cmax increased by 26% and 7% for total and free ezetimibe, respectively. The AUC increased by 27% and 12% for total and free ezetimibe, respectively.
Table 2 describes the effects of co-administered drugs on fenofibric acid systemic exposure. Table 3 describes the effects of co-administered fenofibric acid on other drugs.
| Co-
Administered Drug | Dosage Regimen of
Co-Administered Drug | Dosage Regimen of
Trilipix or Fenofibrate | Changes in
Fenofibric Acid Exposure |
|
| AUC | Cmax | |||
| Lipid-lowering agents | ||||
| Rosuvastatin | 40 mg once daily for 10 days | Trilipix 135 mg once daily for 10 days | ↓2% | ↓2% |
| Atorvastatin | 20 mg once daily for 10 days | Fenofibrate 160 mg1
once daily for 10 days | ↓2% | ↓4% |
| Atorvastatin + ezetimibe | Atorvastatin, 80 mg once daily and ezetimibe, 10 mg once daily for 10 days | Trilipix 135 mg once daily for 10 days | ↑5% | ↑5% |
| Pravastatin | 40 mg as a single dose | Fenofibrate 3 x 67 mg2
as a single dose | ↓1% | ↓2% |
| Fluvastatin | 40 mg as a single dose | Fenofibrate 160 mg1
as a single dose | ↓2% | ↓10% |
| Simvastatin | 80 mg once daily for 7 days | Fenofibrate 160 mg1
once daily for 7 days | ↓5% | ↓11% |
| Anti-diabetic agents | ||||
| Glimepiride | 1 mg as a single dose | Fenofibrate 145 mg1
once daily for 10 days | ↑1% | ↓1% |
| Metformin | 850 mg 3 times daily for 10 days | Fenofibrate 54 mg1
3 times daily for 10 days | ↓9% | ↓6% |
| Rosiglitazone | 8 mg once daily for 5 days | Fenofibrate 145 mg1
once daily for 14 days | ↑10% | ↑3% |
| Gastrointestinal agents | ||||
| Omeprazole | 40 mg once daily for 5 days | Trilipix 135 mg as a single dose fasting | ↑6% | ↑17% |
| Omeprazole | 40 mg once daily for 5 days | Trilipix 135 mg as a single dose with food | ↑4% | ↓2% |
| 1 TriCor (fenofibrate) oral tablet 2 TriCor (fenofibrate) oral micronized capsule |
||||
| Dosage Regimen of
Trilipix or Fenofibrate | Dosage Regimen of
Co-Administered Drug | Change in Co-Administered
Drug Exposure |
||
| Analyte | AUC | Cmax | ||
| Lipid-lowering agents | ||||
| Trilipix 135 mg once daily for 10 days | Rosuvastatin, 40 mg once daily for 10 days | Rosuvastatin | ↑6% | ↑20% |
| Fenofibrate 160 mg1 once daily for 10 days | Atorvastatin, 20 mg once daily for 10 days | Atorvastatin | ↓17% | 0% |
| Fenofibrate 3 x 67 mg2 as a single dose | Pravastatin, 40 mg as a single dose | Pravastatin | ↑13% | ↑13% |
| 3α-Hydroxyl-iso-pravastatin | ↑26% | ↑29% | ||
| Fenofibrate 160 mg1 as a single dose | Fluvastatin, 40 mg as a single dose | (+)-3R, 5S-Fluvastatin | ↑15% | ↑16% |
| Fenofibrate 160 mg1 once daily for 7 days | Simvastatin, 80 mg once daily for 7 days | Simvastatin acid | ↓36% | ↓11% |
| Simvastatin | ↓11% | ↓17% | ||
| Active HMG-CoA Inhibitors | ↓12% | ↓1% | ||
| Total HMG-CoA Inhibitors | ↓8% | ↓10% | ||
| Anti-diabetic agents | ||||
| Fenofibrate 145 mg1 once daily for 10 days | Glimepiride, 1 mg as a single dose | Glimepiride | ↑35% | ↑18% |
| Fenofibrate 54 mg1 3 times daily for 10 days | Metformin, 850 mg 3 times daily for 10 days | Metformin | ↑3% | ↑6% |
| Fenofibrate 145 mg1 once daily for 14 days | Rosiglitazone, 8 mg once daily for 5 days | Rosiglitazone | ↑6% | ↓1% |
| 1 TriCor (fenofibrate) oral tablet 2 TriCor (fenofibrate) oral micronized capsule |
||||
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Trilipix (fenofibric acid)
No carcinogenicity and fertility studies have been conducted with choline fenofibrate or fenofibric acid. However, because fenofibrate is rapidly converted to its active metabolite, fenofibric acid, either during or immediately following absorption both in animals and humans, studies conducted with fenofibrate are relevant for the assessment of the toxicity profile of fenofibric acid. A similar toxicity spectrum is expected after treatment with either Trilipix or fenofibrate.
Fenofibrate
Two dietary carcinogenicity studies have been conducted in rats with fenofibrate. In the first 24-month study, Wistar rats were dosed with fenofibrate at 10, 45, and 200 mg/kg/day, approximately 0.3, 1, and 6 times the maximum recommended human dose (MRHD) of 300 mg fenofibrate daily, equivalent to 135 mg Trilipix daily, based on body surface area comparisons. At a dose of 200 mg/kg/day (at 6 times the MRHD), the incidence of liver carcinomas was significantly increased in both sexes. A statistically significant increase in pancreatic carcinomas was observed in males at 1 and 6 times the MRHD; an increase in pancreatic adenomas and benign testicular interstitial cell tumors was observed at 6 times the MRHD in males. In a second 24-month rat carcinogenicity study in a different strain of rats (Sprague-Dawley), doses of 10 and 60 mg/kg/day (0.3 and 2 times the MRHD) produced significant increases in the incidence of pancreatic acinar adenomas in both sexes and increases in testicular interstitial cell tumors in males at 2 times the MRHD.
A 117-week carcinogenicity study was conducted in rats comparing three drugs: fenofibrate 10 and 60 mg/kg/day (0.3 and 2 times the MRHD, based on body surface area comparisons), clofibrate (400 mg/kg/day; 2 times the human dose), and gemfibrozil (250 mg/kg/day; 2 times the human dose, based on mg/m2 surface area). Fenofibrate increased pancreatic acinar adenomas in both sexes. Clofibrate increased hepatocellular carcinoma and pancreatic acinar adenomas in males and hepatic neoplastic nodules in females. Gemfibrozil increased hepatic neoplastic nodules in males and females, while all three drugs increased testicular interstitial cell tumors in males.
In a 21-month study in CF-1 mice, fenofibrate 10, 45, and 200 mg/kg/day (approximately 0.2, 1, and 3 times the MRHD, based on body surface area comparisons) significantly increased the liver carcinomas in both sexes at 3 times the MRHD. In a second 18-month study at 10, 60, and 200 mg/kg/day, fenofibrate significantly increased the liver carcinomas in male mice and liver adenomas in female mice at 3 times the MRHD.
Electron microscopy studies have demonstrated peroxisomal proliferation following fenofibrate administration to the rat. An adequate study to test for peroxisome proliferation in humans has not been done, but changes in peroxisome morphology and numbers have been observed in humans after treatment with other members of the fibrate class when liver biopsies were compared before and after treatment in the same individual.
Fenofibrate has been demonstrated to be devoid of mutagenic potential in the following tests: Ames, mouse lymphoma, chromosomal aberration and unscheduled DNA synthesis in primary rat hepatocytes.
In fertility studies rats were given oral dietary doses of fenofibrate, males received 61 days prior to mating and females 15 days prior to mating through weaning which resulted in no adverse effect on fertility at doses up to 300 mg/kg/day (10 times the MRHD, based on body surface area comparisons).
14. Clinical Studies
14.1 Severe Hypertriglyceridemia
The effects of fenofibrate on serum triglycerides were studied in two randomized, double-blind, placebo-controlled clinical trials of 147 hypertriglyceridemic patients. Patients were treated for eight weeks under protocols that differed only in that one entered patients with baseline TG levels of 500 to 1500 mg/dL, and the other TG levels of 350 to 500 mg/dL. In patients with hypertriglyceridemia and normal cholesterolemia with or without hyperchylomicronemia, treatment with fenofibrate at dosages equivalent to 135 mg once daily of Trilipix decreased primarily VLDL-TG and VLDL-C. Treatment of patients with elevated TG often results in an increase of LDL-C (Table 4).
| Study 1 | Placebo | Fenofibrate | ||||||
| Baseline TG levels
350 to 499 mg/dL | N | Baseline Mean
(mg/dL) | Endpoint Mean
(mg/dL) | Mean % Change | N | Baseline Mean
(mg/dL) | Endpoint Mean
(mg/dL) | Mean % Change |
| Triglycerides | 28 | 449 | 450 | -0.5 | 27 | 432 | 223 | -46.2* |
| VLDL Triglycerides | 19 | 367 | 350 | 2.7 | 19 | 350 | 178 | -44.1* |
| Total Cholesterol | 28 | 255 | 261 | 2.8 | 27 | 252 | 227 | -9.1* |
| HDL Cholesterol | 28 | 35 | 36 | 4 | 27 | 34 | 40 | 19.6* |
| LDL Cholesterol | 28 | 120 | 129 | 12 | 27 | 128 | 137 | 14.5 |
| VLDL Cholesterol | 27 | 99 | 99 | 5.8 | 27 | 92 | 46 | -44.7* |
| Study 2 | Placebo | Fenofibrate | ||||||
| Baseline TG levels 500 to 1500 mg/dL | N | Baseline Mean (mg/dL) | Endpoint Mean (mg/dL) | Mean % Change | N | Baseline Mean (mg/dL) | Endpoint Mean (mg/dL) | Mean % Change |
| Triglycerides | 44 | 710 | 750 | 7.2 | 48 | 726 | 308 | -54.5* |
| VLDL Triglycerides | 29 | 537 | 571 | 18.7 | 33 | 543 | 205 | -50.6* |
| Total Cholesterol | 44 | 272 | 271 | 0.4 | 48 | 261 | 223 | -13.8* |
| HDL Cholesterol | 44 | 27 | 28 | 5.0 | 48 | 30 | 36 | 22.9* |
| LDL Cholesterol | 42 | 100 | 90 | -4.2 | 45 | 103 | 131 | 45.0* |
| VLDL Cholesterol | 42 | 137 | 142 | 11.0 | 45 | 126 | 54 | -49.4* |
| * = p < 0.05 vs. Placebo | ||||||||
14.2 Primary Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
The effects of fenofibrate at a dose equivalent to Trilipix 135 mg once daily were assessed from four randomized, placebo-controlled, double-blind, parallel-group studies including patients with the following mean baseline lipid values: Total-C 306.9 mg/dL; LDL-C 213.8 mg/dL; HDL-C 52.3 mg/dL; and triglycerides 191.0 mg/dL. Fenofibrate therapy lowered LDL-C, Total-C, and the LDL-C/HDL-C ratio. Fenofibrate therapy also lowered triglycerides and raised HDL-C (Table 5).
| Treatment Group | Total-C (mg/dL) | LDL-C (mg/dL) | HDL-C (mg/dL) | TG (mg/dL) |
| Pooled Cohort | ||||
| Mean baseline lipid values (n = 646) | 306.9 | 213.8 | 52.3 | 191.0 |
| All Fenofibrate (n = 361) | -18.7%* | -20.6%* | +11.0%* | -28.9%* |
| Placebo (n = 285) | -0.4% | -2.2% | +0.7% | +7.7% |
| Baseline LDL-C > 160 mg/dL
and TG < 150 mg/dL | ||||
| Mean baseline lipid values (n = 334) | 307.7 | 227.7 | 58.1 | 101.7 |
| All Fenofibrate (n = 193) | -22.4%* | -31.4%* | +9.8%* | -23.5%* |
| Placebo (n = 141) | +0.2% | -2.2% | +2.6% | +11.7% |
| Baseline LDL-C > 160 mg/dL
and TG ≥ 150 mg/dL | ||||
| Mean baseline lipid values (n = 242) | 312.8 | 219.8 | 46.7 | 231.9 |
| All Fenofibrate (n = 126) | -16.8%* | -20.1%* | +14.6%* | -35.9%* |
| Placebo (n = 116) | -3.0% | -6.6% | +2.3% | +0.9% |
| † Duration of study treatment was 3 to 6 months * p = < 0.05 vs. Placebo |
||||
In a subset of the subjects, measurements of Apo B were conducted. Fenofibrate treatment significantly reduced Apo B from baseline to endpoint as compared with placebo (-25.1% vs. 2.4%, p < 0.0001, n = 213 and 143, respectively).
16. How is Trilipix supplied
Trilipix (fenofibric acid) delayed release capsules 45 mg:
- A reddish brown to orange brown cap and a yellow body imprinted in black ink the number “45”, available in bottles of 90 (NDC 0074-3161-90).
Trilipix (fenofibric acid) delayed release capsules 135 mg:
- A blue cap and a yellow body imprinted in black ink the number “135”, available in bottles of 90 (NDC 0074-3162-90).
Store at 25°C (77°F); excursions permitted to 15°-30°C (59° to 86°F) [See USP controlled room temperature]. Keep out of the reach of children. Protect from moisture.
17. Patient Counseling Information
Patient Counseling
Patients should be advised:
- of the potential benefits and risks of Trilipix.
- not to use Trilipix if there is a known hypersensitivity to fenofibrate or fenofibric acid.
- of medications that should not be taken in combination with Trilipix.
- that if they are taking coumarin anticoagulants, Trilipix may increase their anti-coagulant effect, and increased monitoring may be necessary.
- to continue to follow an appropriate lipid-modifying diet while taking Trilipix.
- to take Trilipix once daily, without regard to food, at the prescribed dose, swallowing each capsule whole.
- to return to their physician’s office for routine monitoring.
- to inform their physician of all medications, supplements, and herbal preparations they are taking and any change to their medical condition. Patients should also be advised to inform their physicians prescribing a new medication that they are taking Trilipix.
- to inform their physician of symptoms of liver injury (e.g., jaundice, abdominal pain, nausea, malaise, dark urine, abnormal stool, pruritus); any muscle pain, tenderness, or weakness; or any other new symptoms.
- not to breastfeed during treatment with Trilipix and for 5 days after the final dose.
© 2024 AbbVie. All rights reserved.
Trilipix and its design are trademarks of AbbVie Inc.
Manufactured for AbbVie Inc., North Chicago, IL 60064, U.S.A. by Fournier Laboratories Ireland Limited, Anngrove, Carrigtwohill Co. Cork, Ireland, or AbbVie LTD, Barceloneta, PR 00617.
November, 2024
NDC 0074-3161-90
Trilipix®
Fenofibric Acid
Delayed Release Capsules 45 mg*
90 Capsules
Rx only abbvie
| TRILIPIX
fenofibric acid capsule, delayed release |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| TRILIPIX
fenofibric acid capsule, delayed release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| TRILIPIX
fenofibric acid capsule, delayed release |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| TRILIPIX
fenofibric acid capsule, delayed release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - AbbVie Inc. (078458370) |
More about Trilipix (fenofibric acid)
- Check interactions
- Compare alternatives
- Reviews (18)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: fibric acid derivatives