Pyridostigmine ER tablets: Package Insert / Prescribing Info
Package insert / product label
Generic name: pyridostigmine bromide
Dosage form: tablet, extended release
Drug class: Cholinergic muscle stimulants
Medically reviewed by Drugs.com. Last updated on Oct 15, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
PYRIDOSTIGMINE BROMIDE extended-release tablets, for oral use
Initial U.S. Approval: 1955
WARNING: RISKS WITH IMPROPER USE OF PYRIDOSTIGMINE BROMIDE
See full prescribing information for complete boxed warning.
After Exposure to Soman, Use Atropine and Pralidoxime
-
Pyridostigmine bromide is for use as a pretreatment for exposure to soman nerve agent. Pyridostigmine bromide alone will not protect against exposure to soman. The efficacy of pyridostigmine bromide is dependent upon the rapid use of atropine and pralidoxime (2-PAM) after soman exposure. (2.1, 5.1)
Always Use Protective Garment(s)
- Primary protection against exposure to chemical nerve agents is the wearing of protective garments. (2.1, 5.1)
Use Pyridostigmine Bromide as Pretreatment Only
Pyridostigmine bromide must not be taken after exposure to soman. If taken immediately before soman exposure (e.g., when the gas attack alarm is given) or at the same time as poisoning by soman, it is not expected to be effective and may exacerbate the effects of a sub-lethal exposure to soman. (2.1, 12.2)
Indications and Usage for Pyridostigmine ER tablets
Pyridostigmine bromide is a cholinesterase inhibitor indicated for pretreatment against the lethal effects of soman nerve agent poisoning in adults. (1)
Pyridostigmine bromide is for use in conjunction with
- Protective garments, including a gas mask, and
- Immediate atropine and pralidoxime therapy at the first sign of nerve agent poisoning. (1)
Pyridostigmine ER tablets Dosage and Administration
- The recommended dosage is 105 mg orally once daily, started at least several hours prior to exposure to soman. Do not take on an empty stomach. Take with a medium-fat medium-calorie meal (e.g., meal with 650 calories, 40% fat) or a high-fat high-calorie meal (e.g., meal with 1,000 calories, 50% fat). Do not take with a low-fat low-calorie meal (e.g., meal with 400 calories, 25% fat) (2.2)
- At the first sign of soman nerve agent poisoning, discontinue pyridostigmine and administer atropine and pralidoxime immediately. (2.2)
- Evaluate use beyond 14 consecutive days in the context of the likelihood of soman exposure. (2.2)
Dosage Forms and Strengths
Extended-release tablets: 105 mg (3)
Contraindications
Warnings and Precautions
- At the first sign of soman poisoning pyridostigmine must be stopped, atropine and 2-PAM must be administered immediately. (5.1)
- Use with caution in persons with increased risk of anticholinergic reactions, such as persons with bronchial asthma, chronic obstructive pulmonary disease, bradycardia, cardiac arrhythmias, beta blocker treatment (increased risk of anticholinergic reactions). (5.2)
- Use with caution in persons with bromide sensitivity. (5.3)
- In case of serious adverse reactions, advise personnel to temporarily discontinue pyridostigmine and seek immediate medical attention. (5.4)
Adverse Reactions/Side Effects
Most common adverse reactions ( ≥ 3% ) are diarrhea, abdominal pain, dysmenorrhea, and twitch. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Mefloquine: Additive effect on gastrointestinal tract and atrial rate. (7.1)
- Anticholinesterase drugs for glaucoma treatment: Additive effect. (7.2)
- Narcotics: Exacerbation of bradycardia possible. (7.3)
- Depolarizing neuromuscular blocking agents: Increased effect. (7.4)
- Non-depolarizing neuromuscular blocking agents: Dose may need to be increased. (7.4)
- Aminoglycoside antibiotics, local and some general anesthetics, antiarrhythmic agents, and other drugs that interfere with neuromuscular transmission should be used cautiously, if at all. (7.4)
- Drugs converted to pantothenic acid (e.g., dexpanthenol): Additive effect. (7.5)
Use In Specific Populations
Renal impairment: Increased risk of side effects; careful dose selection. In persons with renal impairment, renal function monitoring may be useful. (8.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2024
Full Prescribing Information
WARNING: RISKS WITH IMPROPER USE OF PYRIDOSTIGMINE BROMIDE
After Exposure to Soman, Use Atropine and Pralidoxime
- Pyridostigmine bromide is for use as a pretreatment for exposure to soman nerve agent. Pyridostigmine bromide alone will not protect against exposure to soman. The efficacy of pyridostigmine bromide is dependent upon the rapid use of atropine and pralidoxime (2-PAM) after soman exposure [see Dosage and Administration (2.1) and Warnings and Precautions (5.1)].
Always Use Protective Garment(s)
- Primary protection against exposure to chemical nerve agents is the wearing of protective garments including masks, hoods, and overgarments designed specifically for this use. Individuals must not rely solely upon pretreatment with pyridostigmine bromide and on the antidotes atropine and pralidoxime (2-PAM) to provide complete protection from poisoning by soman nerve agent [see Dosage and Administration (2.1)].
Use Pyridostigmine Bromide as Pretreatment Only
- Pyridostigmine bromide must not be taken after exposure to soman. If pyridostigmine bromide is taken immediately before exposure (e.g., when the gas attack alarm is given) or at the same time as poisoning by soman, it is not expected to be effective and may exacerbate the effects of a sub-lethal exposure to soman [see Clinical Pharmacology (12.2)].
FOR MILITARY MEDICAL USE ONLY
1. Indications and Usage for Pyridostigmine ER tablets
Pyridostigmine bromide is indicated for pretreatment against the lethal effects of soman nerve agent poisoning in adults. Pyridostigmine bromide is intended for use in conjunction with protective garments, including a mask. At the first sign of nerve agent poisoning, pyridostigmine bromide should be stopped, and atropine and pralidoxime therapy started immediately.
The evidence for the effectiveness of pyridostigmine bromide as pretreatment against soman-induced toxicity was derived from animal studies alone [see Clinical Studies (14)].
2. Pyridostigmine ER tablets Dosage and Administration
2.1 Important Administration Information
For Military Medical Use Only.
Treatment and Protection for Soman Exposure
Pyridostigmine bromide is for use as a pretreatment for exposure to soman nerve agent (see Treatment Timing). Pyridostigmine bromide alone will not protect against exposure to soman.
Atropine and Pralidoxime
The efficacy of pyridostigmine bromide is dependent upon the rapid use of atropine and pralidoxime (2-PAM) after soman exposure.
Primary Protection
The primary protection against exposure to chemical nerve agents consists of wearing protective garments including masks, hoods, and overgarments designed specifically for this use.
Do not rely solely upon pretreatment with pyridostigmine bromide and the antidotes atropine and pralidoxime to provide complete protection from poisoning by soman nerve agent.
Treatment Timing
Pyridostigmine bromide is to be administered as pretreatment before exposure to soman nerve agent [see Dosage and Administration (2.2)]. If pyridostigmine bromide is taken immediately before exposure (e.g., when the gas attack alarm is given) or at the same time as poisoning by soman, it is not expected to be effective and may exacerbate the effects of a sublethal exposure to soman [see Clinical Pharmacology (12.2)]. Do not take pyridostigmine bromide after exposure to soman.
Administration with Food
Take pyridostigmine bromide 105 mg extended-release tablets with either a medium fat, medium calorie or high fat, high calorie meal to maintain efficacious pyridostigmine levels [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
Administration with Alcohol
Pyridostigmine bromide 105 mg extended-release tablets should not be taken with alcohol [see Clinical Pharmacology (12.3)].
2.2 Recommended Dosage, Administration, and Duration
Dosage
The recommended dosage of pyridostigmine bromide is 105 mg orally once daily with food, started at least several hours prior to exposure to soman. Do not take on an empty stomach. Take with a medium-fat medium-calorie meal (e.g., meal with 650 calories, 40% fat) or a high-fat high-calorie meal (e.g., meal with 1,000 calories, 50% fat). Do not take with a low-fat low-calorie meal (e.g., meal with 400 calories, 25% fat) [see Clinical Pharmacology (12.3)].
Timing and Duration of Treatment
Timing
Pyridostigmine bromide is a pretreatment for exposure to soman nerve agent.
There is no known advantage to taking pyridostigmine bromide just prior to, or concurrent with, soman exposure. According to the mechanism of action of pyridostigmine bromide [see Clinical Pharmacology (12.1, 12.2)], pyridostigmine bromide is effective when it is given sufficiently in advance of soman poisoning to provide a pool of protected enzyme. Therefore, it is expected that pyridostigmine bromide will not be effective if administered just prior to or during exposure to soman.
Duration
At the first sign of nerve agent poisoning, discontinue pyridostigmine bromide and administer treatment with atropine and pralidoxime immediately [see Warnings and Precautions (5.1)].
The benefits and risks of use beyond 14 consecutive days have not been definitively established; therefore, evaluate continued use beyond 14 consecutive days in the context of the likelihood of exposure to soman nerve agent.
Missed Dose
If a dose is missed, take the missed dose as soon as possible. Resume dosing at 24-hour intervals from the time the missed dose was taken. Do not take double or extra doses.
3. Dosage Forms and Strengths
Extended-Release Tablets: 105 mg, peach, oval, coated, unscored, biconvex tablets imprinted with “A2 463” on one side and plain on the other side. A hole may be visible on one side of the tablet.
4. Contraindications
Pyridostigmine bromide extended-release tablets are contraindicated in patients with:
- Mechanical intestinal or urinary obstruction
- Known hypersensitivity to pyridostigmine or other anticholinesterase agents
5. Warnings and Precautions
5.1 Risk of Improper Use of Pyridostigmine Bromide
Risk of Not Stopping Pyridostigmine Bromide and Using Atropine and Pralidoxime in the Event of Soman Exposure
Pyridostigmine bromide is for use as a pretreatment for exposure to soman nerve agent and must not be taken after exposure to soman nerve agent [see Dosage and Administration (2.1, 2.2)]. Pyridostigmine bromide pretreatment offers no benefit against the nerve agent soman unless the nerve agent antidotes atropine and pralidoxime (2-PAM) are administered once symptoms of poisoning appear.
Discontinue pyridostigmine at the first sign of nerve agent poisoning because it may exacerbate the effects of a sub-lethal exposure to soman if taken immediately before exposure (e.g., when the gas attack alarm is given) or at the same time as poisoning by soman [see Clinical Pharmacology (12.2)]. Signs of nerve agent poisoning can include runny nose; watery eyes; small, pinpoint pupils; eye pain; blurred vision; drooling and excessive sweating; cough; chest tightness; rapid breathing; diarrhea; increased urination; confusion; drowsiness; weakness; headache; nausea, vomiting, and/or abdominal pain; slow or fast heart rate; and/or abnormally low or high blood pressure.
Risk of Not Wearing Protective Garments
Pyridostigmine bromide is not the primary protection against exposure to soman nerve agent.
The primary protection against exposure to chemical nerve agents is the wearing of protective garments including masks, hoods, and overgarments designed specifically for this use. Individuals must not rely solely upon pretreatment with pyridostigmine bromide and on the antidotes atropine and pralidoxime (2-PAM) to provide complete protection from poisoning by soman nerve agent.
5.2 Increased Risk of Anticholinergic Adverse Reactions in Individuals with Certain Conditions
Pulmonary Conditions
Drugs that increase cholinergic activity, including pyridostigmine bromide, should be used with caution in persons with bronchial asthma or chronic obstructive pulmonary disease.
Cardiovascular Conditions
Because pyridostigmine bromide increases cholinergic activity, it may have vagotonic effects on heart rate, which can lead to bradycardia or cardiac arrhythmias.
Genitourinary Tract or Gastrointestinal Tract Obstruction
Drugs that increase cholinergic agents, including pyridostigmine bromide, could cause symptoms in persons susceptible to genitourinary tract or gastrointestinal tract obstruction.
Conditions Treated with Beta Adrenergic Receptor Blockers
Pyridostigmine bromide should be used with caution in people being treated for hypertension or glaucoma with beta adrenergic receptor blockers [see Drug Interactions (7.2)].
5.3 Use in Bromide-Sensitive Individuals
Caution should be taken when administering pyridostigmine bromide to individuals with known bromide sensitivity. A skin rash may be observed, which usually subsides upon discontinuance of the medication. The risks and benefits of administration must be weighed against the potential for rash or other adverse reactions in these individuals.
5.4 Serious Adverse Reactions, Such as Difficulty Breathing, Severe Dizziness, Loss of Consciousness
Pyridostigmine bromide can cause serious adverse reactions such as difficulty breathing, severe dizziness, or loss of consciousness. If these adverse reactions occur, patients should temporarily discontinue use of pyridostigmine bromide and seek immediate medical attention. Personnel should report serious adverse events to their commander and responsible medical officer.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed elsewhere in the labeling:
- Risk of Improper Use of Pyridostigmine Bromide [see Warnings and Precautions (5.1)]
- Individuals at Increased Risk of Anticholinergic Adverse Reactions [see Warnings and Precautions (5.2)]
- Use in Bromide-Sensitive Individuals [see Warnings and Precautions (5.3)]
Serious Adverse Reactions, Such as Difficulty Breathing, Severe Dizziness, Loss of Consciousness [see Warnings and Precautions (5.4)]
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The adverse reactions to pyridostigmine bromide are typically of two varieties: muscarinic and nicotinic. Muscarinic adverse reactions include abdominal cramps, bloating, flatulence, diarrhea, emesis, increased peristalsis, nausea, hypersalivation, urinary incontinence, increased bronchial secretion, diaphoresis, miosis, and lacrimation. Nicotinic adverse reactions are comprised chiefly of muscle cramps, fasciculations, and weakness.
In a controlled study of 90 healthy volunteers comparing immediate-release pyridostigmine bromide 30 mg every 8 hours, which provides exposures comparable to pyridostigmine bromide 105 mg extended-release tablets once daily, to placebo for 21 days, the adverse reactions that were reported in 2% or more of subjects is included in Table 1. The most common adverse reactions (≥ 3%) are diarrhea, abdominal pain, dysmenorrhea, and twitch.
Table 1: Incidence of Adverse Reactions ≥ 2%
|
Adverse Reaction |
% |
% |
|
Diarrhea |
7 |
0 |
|
Abdominal Pain |
7 |
0 |
|
Dysmenorrhea |
5 |
0 |
|
Twitch |
3 |
0 |
|
Myalgia |
2 |
0 |
|
Dry Skin |
2 |
0 |
|
Urinary Frequency |
2 |
0 |
|
Epistaxis |
2 |
0 |
|
Amblyopia |
2 |
0 |
|
Hypesthesia |
2 |
0 |
|
Neck pain |
2 |
0 |
Other less common adverse reactions seen during controlled and uncontrolled clinical trials for pyridostigmine include the following:
- Pulmonary: Exacerbation of acute bronchitis and asthma
- Cardiovascular: Elevated blood pressure, decreased heart rate (4-6 beats per minute), chest tightness
- Eyes: Change in vision, eye pain
- Neurologic: Headache, hypertonia, difficulty in concentrating, confusion, disturbed sleep, tingling of extremities, numbness of the tongue
- Skin: Increased sweating, rash, alopecia
- Digestive: Vomiting, borborygmi, nausea, bloating, flatulence
- General: Warm sensation, lethargy/drowsiness, depressed mood
During safety studies at the recommended dosage of immediate release pyridostigmine bromide 30 mg every 8 hours, which provides exposures comparable to pyridostigmine bromide 105 mg extended-release tablets once daily, there were two reports of loss of consciousness, one of which also included urinary and fecal incontinence, stiffness of the upper torso and arms, post-syncopal skin pallor, post-syncopal confusion, and post-syncopal weakness (suggesting a seizure event).
Central Nervous System Adverse Reactions
Pyridostigmine bromide is a quaternary ammonium compound and does not readily cross the blood-brain barrier. Compared to the peripheral effects of pyridostigmine bromide, central nervous system manifestations are less frequent and less serious, primarily consisting of headache and vertigo, with minor and clinically insignificant changes in heart rate, blood pressure, and respiratory function.
Related/similar drugs
7. Drug Interactions
7.1 Mefloquine
Concomitant use of mefloquine and pyridostigmine bromide may have additive effects on the gastrointestinal tract, the most common of which is loose bowels. Additive effects on the atrial rate may also occur with concomitant use of mefloquine and pyridostigmine bromide.
7.2 Other Anticholinesterase Drug
Concomitant use of anticholinesterase drugs used in the treatment of glaucoma and pyridostigmine bromide may have an additive effect that may cause or exacerbate problems with night vision.
7.3 Narcotics
The bradycardia associated with the use of narcotics may exacerbate pyridostigmine-induced bradycardia.
7.4 Drugs that Interfere with Neuromuscular Transmission
Particular caution should be observed in the administration of depolarizing neuromuscular blocking agents (e.g., succinylcholine) during surgery since the degree of neuromuscular blockade that ensues may be enhanced by previously administered pyridostigmine bromide. Doses of non-depolarizing neuromuscular blocking agents (e.g., pancuronium bromide) may need to be increased in patients previously administered pyridostigmine bromide. Atropine antagonizes the muscarinic effects of pyridostigmine bromide, and this interaction is utilized to counteract the muscarinic symptoms of pyridostigmine bromide toxicity. Anticholinesterase agents are sometimes effective in reversing neuromuscular block induced by aminoglycoside antibiotics. However, aminoglycoside antibiotics, local and some general anesthetics, antiarrhythmic agents, and other drugs that interfere with neuromuscular transmission should be used cautiously, if at all, during treatment with pyridostigmine bromide.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Available data from case reports and case series over decades of use with pyridostigmine bromide have not identified a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Treatment with pyridostigmine bromide for soman nerve agent poisoning during pregnancy should not be delayed because exposure to soman during pregnancy may increase the risk of maternal and fetal morbidity and mortality.
In animal studies, oral administration of pyridostigmine during organogenesis resulted in adverse developmental effects (increased embryofetal mortality and fetal structural abnormalities), at clinically relevant doses (see Data).
The background risk for major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in the clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Oral administration of pyridostigmine to pregnant rats during organogenesis resulted in increases in embryofetal deaths and incidences of fetal skeletal variations at the highest dose tested (30 mg/kg/day), which was also maternally toxic. A slight increase in the incidence of hydronephrosis was observed at all dose levels (lowest dose tested was 3 mg/kg/day). A no-effect dose for adverse effects on embryofetal development in the rat was not identified. The lowest dose tested (3 mg/kg/day) is less than the recommended human dose (105 mg) on a body surface area (mg/m2) basis.
Oral administration of pyridostigmine to pregnant rabbits during organogenesis resulted in an increase in the incidence of visceral variations at the highest dose tested (45 mg/kg/day), which was also maternally toxic. An increased incidence of blood vessel variations was observed at all doses (lowest dose tested was 5 mg/kg/day). A no-effect dose for adverse effects on embryofetal development in the rabbit was not identified. The lowest dose tested (5 mg/kg/day) is less than the recommended human dose on a body surface area (mg/m2) basis.
8.2 Lactation
Risk Summary
Pyridostigmine bromide is present in human milk. Based on limited data, pyridostigmine bromide was not detected in the plasma of infants who were breastfed by mothers taking pyridostigmine bromide. There are no data on the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for pyridostigmine bromide and any potential adverse effects on the breastfed infant from pyridostigmine bromide or from the underlying maternal condition.
8.5 Geriatric Use
Clinical studies of pyridostigmine bromide did not contain sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function [see Clinical Pharmacology (12.3)], care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Renal Impairment
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Caution should be observed, and dosage be selected carefully, when administering pyridostigmine bromide to patients with impaired renal function. [see Clinical Pharmacology (12.3)]. It may be useful to monitor renal function.
10. Overdosage
Overdosage of pyridostigmine bromide may result in cholinergic crisis, a state characterized by increasing muscle weakness that, through involvement of the muscles of respiration, may lead to death. Overdosage with pyridostigmine bromide must be differentiated from the acute manifestations of nerve agent poisoning, which may also be characterized by a cholinergic crisis. Atropine should be used to treat pyridostigmine bromide overdosage.
Extremely high doses of pyridostigmine bromide may also produce central nervous system (CNS) symptoms of agitation, restlessness, confusion, visual hallucinations, and paranoid delusions. Electrolyte abnormalities, possibly resulting from high serum bromide concentrations, also have been reported. Death may result from cardiac arrest or respiratory paralysis and pulmonary edema.
In the treatment of pyridostigmine bromide overdosage, maintaining adequate respiration is of primary importance. Tracheostomy, bronchial aspiration, and postural drainage may be required to maintain an adequate airway; respiration can be assisted mechanically if required. Supplemental oxygen may be necessary. Pyridostigmine bromide should be discontinued immediately and 1 mg to 4 mg of atropine sulfate administered intravenously. Additional doses of atropine may be given every 5 to 30 minutes as needed to control muscarinic symptoms. Atropine overdosage should be avoided, as tenacious secretions and bronchial plugs may result. It should be kept in mind that unlike muscarinic effects, the skeletal muscle effects and consequent respiratory paralysis (nicotinic effects), which can occur following pyridostigmine overdosage, are not alleviated by atropine.
11. Pyridostigmine ER tablets Description
Pyridostigmine bromide is an orally active, reversible cholinesterase inhibitor. Pyridostigmine Bromide is a white or almost white, crystalline, deliquescent powder, very soluble in water and alcohol, and practically insoluble in ether.
CAS registration number is 101-26-8.
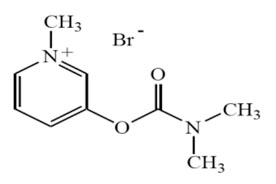
Pyridostigmine bromide molecular formula is C9H13BrN2O2, with a molecular weight of 261.12. It is designated chemically as 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate and its structural formula is:
Pyridostigmine bromide extended-release tablets contain 105 mg of pyridostigmine bromide (equivalent to 72.87 mg of pyridostigmine base) for oral administration. The inactive ingredients included in the tablet formula are ammonia methacrylate copolymer, ammonium hydroxide, black iron oxide, calcium carbonate, colloidal silicon dioxide, crospovidone, hydroxypropyl cellulose, hypromellose, iron oxide red, iron oxide yellow, magnesium stearate, mannitol, propylene glycol, shellac glaze, sodium bicarbonate, succinic acid, talc, titanium dioxide, and triethyl citrate.
12. Pyridostigmine ER tablets - Clinical Pharmacology
12.2 Pharmacodynamics
The mechanism of soman-induced death is reasonably well understood. Death is believed to result primarily from respiratory failure due to irreversible inhibition of the enzyme acetylcholinesterase and the consequent increase in the level of the neurotransmitter acetylcholine 1) at nicotinic receptors at the neuromuscular junction, resulting in pathological stimulation and ultimate failure of the muscles of respiration, 2) at muscarinic receptors in secretory glands and smooth muscle, resulting in excessive respiratory secretions and bronchoconstriction, and 3) at cholinergic receptors in the brain, resulting in central respiratory depression.
The effect of pyridostigmine is presumed to result from its reversible inhibition of a critical number of acetylcholinesterase active sites in the peripheral nervous system, protecting them from irreversible inhibition by soman. (Pyridostigmine bromide is not thought to enter the brain in significant amounts.) When the pyridostigmine bromide-induced inhibition of the enzyme is subsequently reversed, there is a small residual amount of enzyme activity that is adequate to sustain life (provided atropine and 2-PAM are subsequently administered). An implication of this presumed mechanism is that it is not helpful to give pyridostigmine bromide either just before or during exposure to soman [see Dosage and Administration (2.1, 2.2)].
12.3 Pharmacokinetics
Absorption
Pyridostigmine bromide is poorly absorbed from the gastrointestinal tract with an absolute bioavailability of 10% to 20%. Following a single oral dose of 105 mg pyridostigmine bromide extended-release tablet in the fed state, the median Tmax was 10.00 (5.00 – 18.00) hours with medium fat, medium calorie meal and 16.00 (5.00 – 20.00) hours with high fat, high calorie meal.
Effect of Food
The pharmacokinetic parameters of pyridostigmine bromide 105 mg extended-release tablets showed a significant food effect in regard to fasted vs. fed and to the types of meals consumed. Single oral doses were administered to healthy volunteers (N=32) under four different conditions as described below:
- Fasted state
- Low Fat, Low Calorie (LFLC): ~450-500 Kcal; fat ~25%
- Medium Fat, Medium Calorie (MFMC): ~600-650 Kcal; fat ~40%
- High Fat, High Calorie (HFHC): ~800-1000 Kcal; fat ~50%
Following administration of 105 mg pyridostigmine bromide extended-release tablets, the oral bioavailability is greater when taken with food than when taken in the fasted state. Under fed conditions, the exposure was greatest with HFHC meal compared to LFLC meal. Peak of exposure and overall exposure were similar with MFMC and HFHC meals.
The exposure is greatest following a HFHC meal, with a 55% higher Cmax and 231% higher AUC compared to the fasted state; a 32% higher Cmax and 89% higher AUC compared to LFLC meal.
Compared to HFHC and MFMC meal, the pyridostigmine exposure is lower during the second half of the proposed 24-hour dosing interval under fasted or LFLC meal condition.
Table 2 and Figure 1 below show pyridostigmine concentration-time profile and summary of pharmacokinetic (PK) parameters, respectively, after single dose administration of 105 mg pyridostigmine bromide extended-release tablet under fasted condition, or with food of different fat and calorie contents [see Dosage and Administration (2.1, 2.2)].
Figure 1: Effect of Food on Concentration-Time Profile of Pyridostigmine Bromide 105 mg Extended-Release Tablets
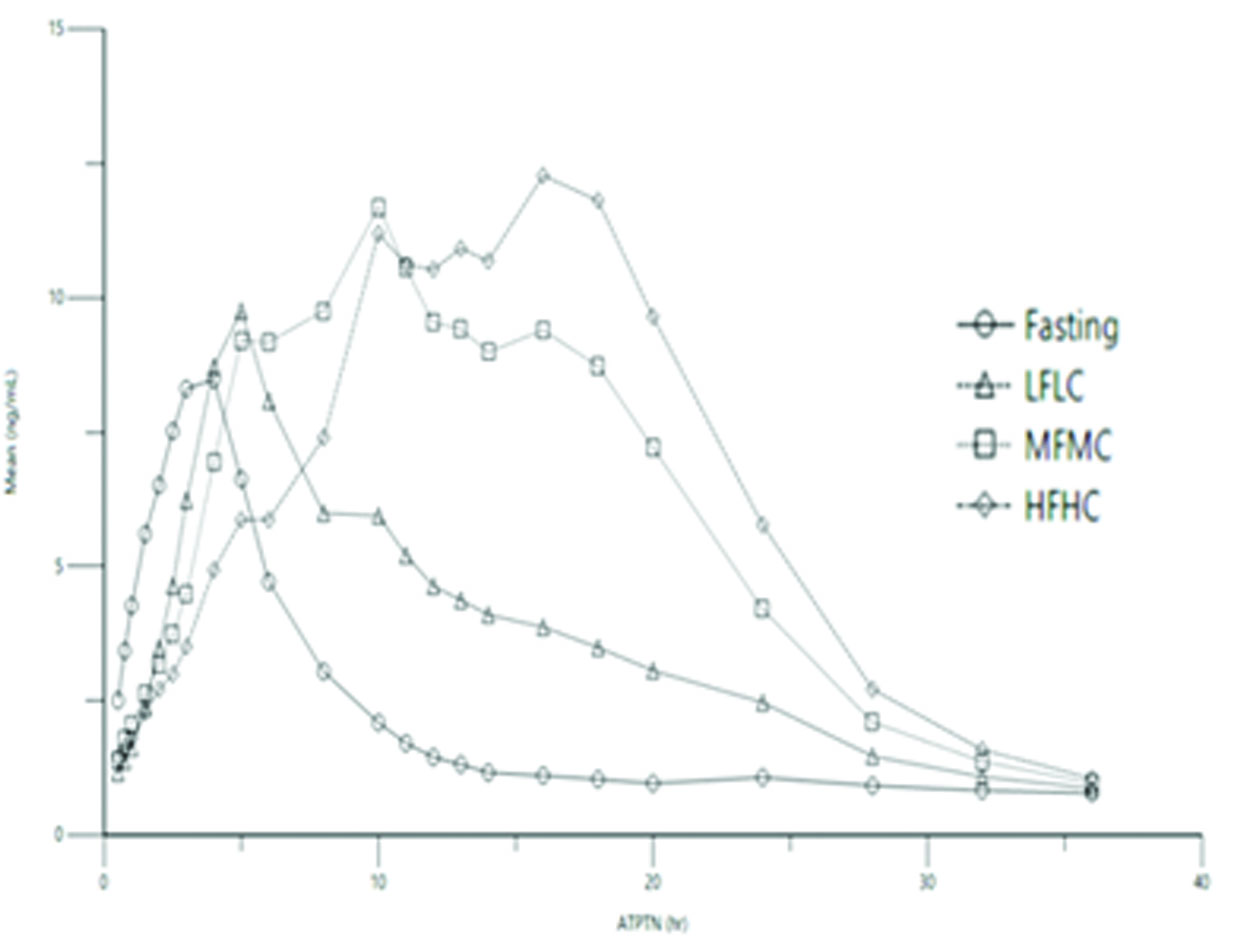
LFLC=low fat, low calorie; MFMC=medium fat, medium calorie; HFHC=high fat, high calorie
Table 2: Summary of Food Effect on PK Parameters of Pyridostigmine Bromide 105 mg Extended-Release Tablets
|
PK Parameters |
Fasted |
Low Fat/ Low Calorie |
Medium Fat/ Medium Calorie |
High Fat/ High Calorie |
|
Mean [SD] |
Mean [SD] |
Mean [SD] |
Mean [SD] |
|
|
Cmax(ng/mL) |
9.30 [4.16] |
10.91 [3.55] |
13.76 [3.72] |
14.43 [3.18] |
|
Tmax* (hours) |
4.00 [1.00 – 5.00] |
5.00 [4.00 – 20.00] |
10.0 [5.00 – 18.00] |
16.00 [5.00 – 20.00] |
|
AUClast (ng.h/L) |
68.90 [33.31] |
120.40 [82.38] |
205.11 [81.09] |
228.15 [59.99] |
|
AUCinf (ng.h/L) |
77.22 [40.5] |
136.45 [88.53] |
215.81 [81.30] |
236.18 [61.35] |
|
T1/2* (hours) |
6.71 [4.15] |
7.39 [5.14] |
5.43 [1.40] |
5.31 [1.51] |
* Arithmetic mean (standard deviation) except Tmax where it is median (minimum, maximum).
Effect of Alcohol
In an in vitro dissolution study where pyridostigmine bromide 105 mg extended-release tablets were assessed in the presence of 0.1 mol/L standardized hydrochloric acid solution (0.1 N HCl) with 0%, 5%, 20%, and 40% ethanol demonstrated that 20% and 40% alcohol can cause faster release of pyridostigmine
[see Dosage and Administration (2.1)].
Distribution
The volume of distribution was about 19 ± 12 liters, indicating that pyridostigmine bromide distributes into tissues. No information on protein binding of pyridostigmine bromide is available.
Elimination
Metabolism
Pyridostigmine bromide undergoes hydrolysis by cholinesterases and is metabolized in the liver.
Excretion
Pyridostigmine bromide is excreted in the urine as both unchanged drug and its metabolites. The systemic clearance of pyridostigmine bromide is 830 mL/min and the elimination half-life of pyridostigmine bromide is approximately 5.6 hours.
Specific Populations
Patients with Renal Impairment
In anephric patients (n = 4), the elimination half-life increased 3-fold and the systemic clearance decreased by 75% [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
No information is available on the pharmacokinetics of pyridostigmine bromide in hepatic impaired patients.
Male and Female Patients
The clearance of pyridostigmine bromide is not influenced by gender.
Geriatric Patients
In a pyridostigmine bromide study in elderly subjects (71 to 85 years of age), the elimination half-life of pyridostigmine and volume of distribution (central and steady-state) were comparable with younger subjects (21 to 51 years of age). However, the systemic plasma clearance was 30% lower in the elderly [see Use in Specific Populations (8.5)].
13. Nonclinical Toxicology
13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility
Carcinogenesis
Studies to evaluate the carcinogenic potential of pyridostigmine bromide have not been conducted.
Mutagenicity
Pyridostigmine bromide was negative in in vitro (bacterial reverse mutation, mammalian gene mutation in Chinese hamster ovary (CHO) cells, clastogenicity in CHO cells) and in vivo (mouse micronucleus) assays. Pyridostigmine bromide was mutagenic and clastogenic in an in vitro mammalian gene mutation assay in mouse lymphoma cells, in the presence of metabolic activation.
Impairment of Fertility
Pyridostigmine bromide did not impair fertility in male or female rats given oral doses of up to 45 mg/kg/day (5 times the recommended human daily dose (105 mg) on a mg/m2 basis) beginning at 10 (males) or 2 (females) weeks prior to mating.
14. Clinical Studies
The effectiveness of pyridostigmine bromide as a pretreatment against the lethal effects of soman nerve agent poisoning has not been determined in humans because adequate and well-controlled field trials have not been feasible and inducing soman nerve agent poisoning in humans to study the drug’s efficacy is not ethical. Therefore, the effectiveness of pyridostigmine bromide as a pretreatment for against the lethal effects of soman nerve agent poisoning was established based on the results of the following adequate and well-controlled animal efficacy studies.
Evidence of the effectiveness of pyridostigmine bromide as a pretreatment for soman poisoning was obtained from studies in animals alone, because it is unethical to perform such studies in humans. While the results of these animal studies cannot be extrapolated to humans with certainty, the extrapolation is supported by the reasonably well understood pathophysiologic mechanisms of the toxicity of soman and the mechanism of the protective effect of pyridostigmine bromide pretreatment, as examined in various animal species. In addition, the results of these animal studies establish that pyridostigmine bromide is reasonably likely to produce clinical benefit in humans. The section below explains the current understanding of the mechanism of soman toxicity and the beneficial effect of pyridostigmine bromide pretreatment, as well as the basis for extrapolating the animal findings to humans.
Pyridostigmine bromide pretreatment has been shown in animals to decrease the lethality of the soman nerve agent, provided atropine and pralidoxime (2-PAM) are administered immediately after exposure to soman.
Rhesus monkeys were given oral doses of pyridostigmine bromide every 8 hours for a total of 6 doses and were challenged with soman given intramuscularly 5 hours after the last pyridostigmine bromide dose. Two dosage groups of pyridostigmine bromide were used: a low-dose group given 1.2 mg/kg for all 6 doses and a high-dose group given 1.2 and 1.8 mg/kg for the first and second doses, respectively, and 2.4 mg/kg for the final 4 doses. These animals were also given atropine and 2-PAM after exposure to soman. An untreated control group and a group given atropine and 2-PAM (but not pyridostigmine bromide) were also used. The primary endpoint in this study was a decrease in the lethality of soman expressed as an increase in the LD50 (the dose of soman that killed 50% of the animals). The atropine/2-PAM control group showed a small but statistically significant 1.6-fold increase in the soman LD50 compared to the untreated control group. The groups given pyridostigmine bromide as well as atropine and 2-PAM showed increases in the soman LD50 of at least 40-fold compared to the untreated control group and at least 25-fold compared to the atropine/2-PAM group. The two dose levels of pyridostigmine bromide showed similar effectiveness.
Additional studies in rhesus monkeys and guinea pigs also showed effectiveness of pyridostigmine bromide (in the presence of post-soman administration of atropine and 2-PAM). The magnitude of effect in guinea pigs was smaller than that in monkeys (soman LD50 increased 4 to 7-fold compared to untreated control and 2- to 4-fold compared to atropine/2-PAM alone).
Pyridostigmine bromide produced only small and inconsistent effects in studies in rats, mice, and rabbits. It is thought that the effect of pyridostigmine bromide in rats and mice is masked by high blood levels of the enzyme carboxylesterase, which eliminates soman from blood and makes those species highly resistant to soman. In a study in which rats were given an inhibitor of carboxylesterase, pretreatment with pyridostigmine bromide plus atropine and 2-PAM increased the LD50 of soman 8.5-fold compared to untreated controls. Humans have little or no carboxylesterase in blood.
Animal studies have shown that pyridostigmine bromide pretreatment was effective only when animals were given atropine and 2-PAM after exposure to soman.
16. How is Pyridostigmine ER tablets supplied
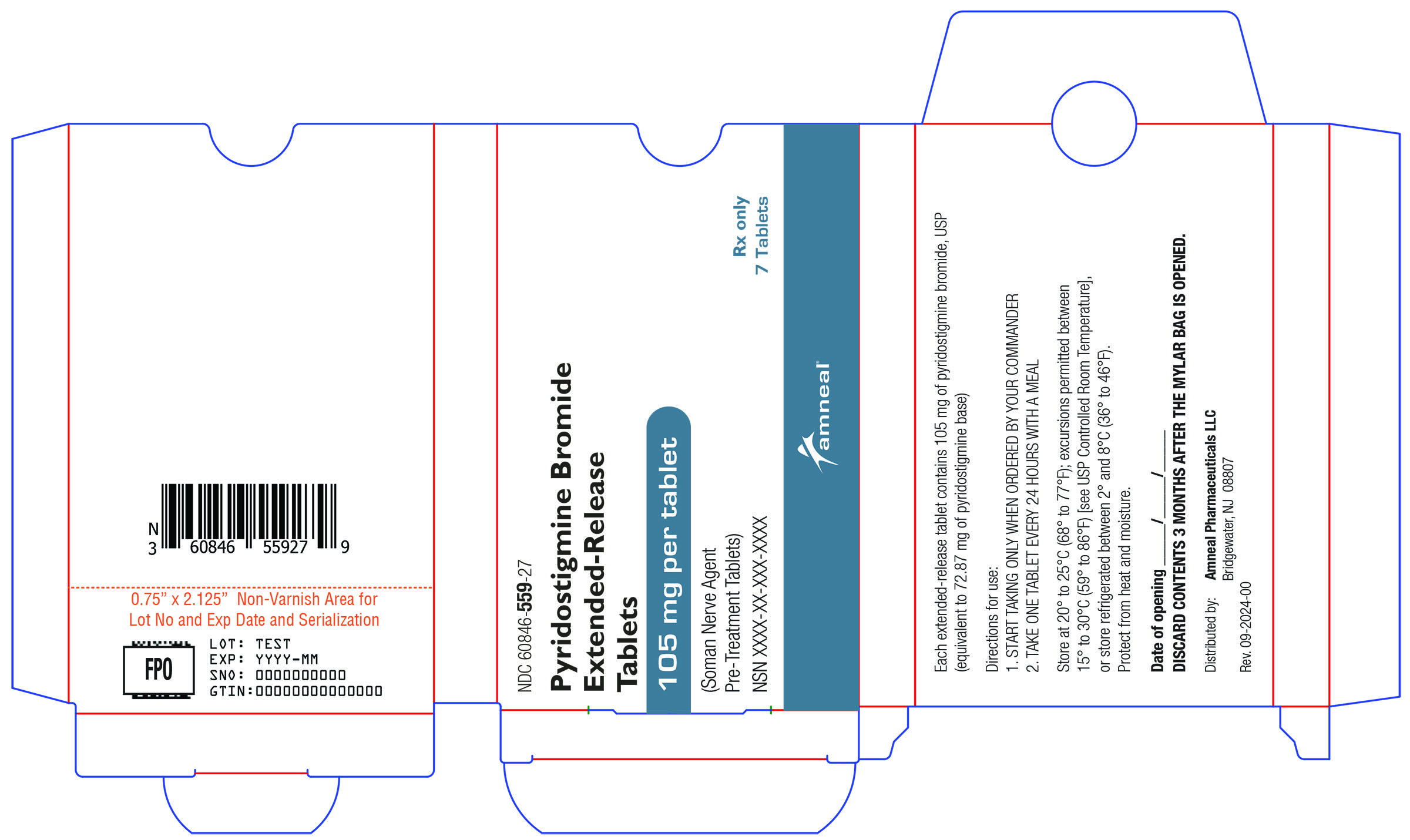
16.1 How Supplied
Pyridostigmine bromide extended-release tablets, 105 mg, are supplied as oval, peach, coated, unscored, biconvex tablets imprinted with “A2 463” on one side and plain on the other side. A hole may be visible on one side of the tablet.
They are available as follows:
Mylar bag containing 10 blister cartons. Each blister carton contains seven (7) tablets.
NDC 60846-559-10.
16.2 Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature], or store refrigerated between 2°C and 8°C (36°F to 46°F). Protect from heat and moisture. Discard contents of blister pack 3 months after the mylar bag is opened.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Indication and Conditions of Use
Advise personnel of the following:
- Pyridostigmine bromide is approved as a pretreatment against the lethal effects of the chemical nerve agent soman (GD). Pyridostigmine bromide has not been approved for use against other chemical nerve agents including Sarin (GB), Tabun (GA), and VX [see Indications and Usage (1)]. The approval is based on safety studies in humans and effectiveness studies conducted in animals. It is not ethical to do effectiveness studies in humans. Human studies would require exposing people to the deadly effects of nerve agents, risking poisoning them or even killing them. Studies in monkeys and guinea pigs show that pretreatment with pyridostigmine bromide makes the antidotes (atropine and 2-PAM) work better against soman (GD) [see Clinical Studies (14)].
- The main protection against chemical weapons is the chemical protective mask and battle dress overgarments [see Dosage and Administration (2.1, 2.2) and Warnings and Precautions (5.1)].
- Pyridostigmine bromide is used as a pretreatment against a soman nerve agent attack. Based on the animal studies, it is thought that any potential benefits from use of pyridostigmine bromide occur only if:
- It is taken at least several hours before, but not right before, exposure to the nerve agent soman. If it is taken right before (when the nerve gas attack alarm is given) or during nerve agent exposure, it may not work and may make the effects of soman worse.
- Atropine and 2-PAM are used when symptoms of nerve agent poisoning occur. [see Dosage and Administration (2.1, 2.2)].
Dosage and Administration [see Dosage and Administration (2.1, 2.2)]
Inform personnel the following:
- The chain of command will instruct when it is time to take pyridostigmine bromide, based on the threat of exposure to soman nerve agents.
- Take one tablet orally once daily, with food, until the chain of command instructs to stop taking pyridostigmine bromide. Do not take on an empty stomach. Take with a medium fat, medium calorie meal (e.g., meal with 650 calories, 40% fat) or a high fat, high calorie meal (e.g., meal with 1,000 calories, 50% fat). Do not take the tablet with a low fat, low calorie meal (e.g., meal with 400 calories, 25% fat).
- Take pyridostigmine bromide only as prescribed.
- If a dose is missed, take the missed dose as soon as possible. Do not take double or extra doses.
- There is no known advantage to taking extra pyridostigmine bromide right before soman exposure.
- Discontinue pyridostigmine bromide after nerve agent exposure has occurred, and instead:
- Persons experiencing most or all of the MILD symptoms of nerve agent poisoning (runny nose; watery eyes; small, pinpoint pupils; eye pain; blurred vision; drooling and excessive sweating; cough; chest tightness; rapid breathing; diarrhea; increased urination; confusion; drowsiness; weakness; headache; nausea, vomiting, and/ or abdominal pain; slow or fast heart rate; and/or abnormally low or high blood pressure) should IMMEDIATELY AVOID INHALATION (hold their breath) AND PUT ON THEIR PROTECTIVE MASK.
- Then atropine and 2-PAM must be administered.
- Contact their unit medical officer if adverse reactions from pyridostigmine bromide continue and limit duty performance.
Risks in Individuals with Certain Conditions [see Contraindications (4), Warnings and Precautions (5.2), and Use in Specific Populations (8.1)]
Instruct personnel to inform their healthcare provider before taking pyridostigmine bromide if they:
- Are pregnant
- Have asthma
- Are allergic to bromide, pyridostigmine, or anticholinesterase drugs
- Take a beta blocker (a medicine to treat, e.g., high blood pressure)
- Have high eye pressure (glaucoma)
- Have any other medical condition, including heart problems, genitourinary or gastrointestinal obstruction, or reflux esophagitis (GERD)
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 10-2024-00
Important Information
Rx Only
PYRIDOSTIGMINE BROMIDE extended-release tablets 105 mg
Protection Against Soman Nerve Agent Poisoning
Pyridostigmine bromide (PB) is approved for pretreatment against the chemical nerve agent Soman (GD). PB has not been approved for use against other chemical nerve agents including Sarin (GB), Tabun (GA) and VX. Nerve agents work by making your muscles weak. They can make you lose control of your muscles. You can die if your breathing muscles are paralyzed.
Your main protection against chemical weapons is your chemical protective mask and battle dress overgarment. You also have other items to help you ifyou are exposed to chemical warfare agents. These items are:
- Two antidotes (atropine and 2-PAM)
- PB is approved as a pretreatment against a soman nerve agent attack. The approval is based on safety studies in humans and effectiveness (how well it works) studies conducted in animals. The FDA has approved PB based only on animal studies of effectiveness because it is not ethical to do these studies in humans. Human studies would require exposing people to the deadly effects of nerve agents, risking poisoning them or even killing them. Studies in monkeys and guinea pigs show that pretreatment with PB makes the antidotes (atropine and 2-PAM) work better against soman (GD). PB pretreatment in animals has not been shown to make the antidotes (atropine and 2-PAM) work better against other nerve agents. Based on the animal studies of whether PB works against soman, it is thought that any potential benefits from use of PB occur only if:
- PB is taken with enough time before, but not right before, exposure to the nerve agent soman. (If PB is taken right before (when the nerve gas attack alarm is given) or during nerve agent exposure, it may not work and may make the effects of soman worse.)
- Atropine and 2-PAM are used when symptoms of nerve agent poisoning occur.
How To Take Your PB
- Your chain of command will tell you when it is time to take PB. This decision will be based on the threat of exposure to soman nerve agent.
- You must take 1 tablet of PB by mouth every 24 hours with a meal that is medium-fat medium calorie (e.g., 650 calories and 40% fat) or high-fat high calorie (e.g., 1000 calories and 50% fat). Do not take PB on an empty stomach. Take daily until your chain of command tells you to stop taking PB. PB should not be taken with alcohol.
- Do not take PB more often than you are told. If you miss a dose of PB, take the missed dose as soon as possible, then take the next dose 24 hours after taking the missed dose. This will put you back on schedule. Do not double up on your dose or take extra doses of PB.
- There is no known advantage to taking extra PB right before soman exposure.
- Do not continue to take PB after nerve agent exposure has occurred, instead:
- If you experience most or all of the Mild symptoms of nerve agent poisoning, you should Immediately hold your breath (Do Not Inhale)andPut On Your Protective Mask. Then administer atropine and 2-PAM.
- Contact your unit medical officer if side effects from PB continue and limit duty performance.
Who should not take PB?
Do not Take PB if you:
- have a history of bowel or bladder blockage (obstruction)
- are allergic to anticholinesterase medicines (certain drugs used during surgery like physostigmine, edrophonium, neostigmine, and ambenonium)
Tell your doctor or medic before taking PB if you:
- are pregnant
- have asthma
- are allergic to bromide
- take blood pressure medicine
- have high eye pressure (glaucoma)
- have problems with your kidneys (renal impairment)
Also, tell your doctor about all your other medical conditions you may have including heart problems, or reflux esophagitis (GERD).
Side Effects
|
|
If your side effects do not go away, see your unit doctor or medic.
This is not a complete list of symptoms that may occur. See your unit doctor right away if your side effects are very bad or for any symptoms that concern you.
About your rights and welfare: DOD may collect information on the use of PB to help decide how best to protect deployed forces in the future. Information that identifies you will remain private (confidential). However, the FDA may review any data collected by DOD for the purpose of evaluating PB. Direct questions about your rights and welfare to your unit medical officer, or e-mail questions to hsrrb@det.amedd.army.mil.
For more information about PB: Talk to your unit medical officer or medic. You can also e-mail questions about PB directly to the U.S. Army Medical Research and Materiel Command at address hsrrb@det.amedd.army.mil.
Manufactured and Distributed by:
Amneal Specialty, a division of Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807 Issued 10/2024
| PYRIDOSTIGMINE BROMIDE
pyridostigmine bromide tablet, extended release |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Amneal Pharmaceuticals LLC (123797875) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Amneal Complex Products Research LLC | 117580013 | analysis(60846-559) , manufacture(60846-559) | |
Frequently asked questions
- Can Mestinon be used to treat Postural Tachycardia Syndrome (POTS)?
- How does Mestinon help with myasthenia gravis?
- Can I stop taking Mestinon (pyridostigmine)?
- What's the mechanism for Mestinon (pyridostigmine)?
- Is Mestinon an Immunosuppressant?
More about pyridostigmine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (59)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: cholinergic muscle stimulants
- Breastfeeding
- En español
Patient resources
Professional resources
- Pyridostigmine monograph
- Pyridostigmine (FDA)
- Pyridostigmine Bromide Oral Solution (FDA)
- Pyridostigmine Bromide Solution (FDA)
- Pyridostigmine Oral Solution (FDA)