Pigment Control Program - Hydroquinone: Package Insert / Prescribing Info
Package insert / product label
Generic name: hydroquinone
Dosage form: kit
Drug class: Topical depigmenting agents
Medically reviewed by Drugs.com. Last updated on Jan 8, 2025.
On This Page
Pigment Control Program - Hydroquinone Description
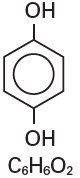
Hydroquinone is 1,4-benzendiol, with a chemical formula of C6H6O2 and a molecular weight of 110.11.
The structural formula is:
Each gram of Pigment Control Creme (Hydroquinone USP, 4%) contains Hydroquinone USP 40 mg/gm in a base of Purified Water, Ascorbic Acid, Ascorbyl Palmitate, Beta-Glucan, Caprylyl Glycol, Cetyl Alcohol, Chlorphenesin, Dioscorea Villosa (Wild Yam) Root Extract, Disodium EDTA, Glycerin, Glycolic Acid, Phenoxyethanol, Quillaja Saponaria Bark Extract, Smilax Aristolochiifolia Root Extract, Sodium Hydroxide, Sodium Lauryl Sulfate, Sodium Metabisulfite, Sodium Sulfite, Stearyl Alcohol, Tocopheryl Acetate, Yucca Schidigera Root Extract.
Pigment Control Program - Hydroquinone - Clinical Pharmacology
Topical application of hydroquinone produces a reversible depigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to 3,4-dihydroxyphenylalanine (DOPA) and suppression of other melanocyte metabolic processes. Exposure to sunlight or ultraviolet light will cause re-pigmentation of bleached areas, which may be prevented by the use of the sunscreen agents.
Indications and Usage for Pigment Control Program - Hydroquinone
Pigment Control Creme is indicated in the gradual bleaching of hyperpigmentation, skin conditions such as chloasma, melasma, freckles, senile lentigines, and other unwanted areas of melanin hyperpigmentation.
Contraindications
Prior history of sensitivity or allergic reaction to this product or any of its ingredients. The safety of topical Hydroquinone use during pregnancy or in children (12 years and under) has not been established.
Warnings
Hydroquinone is a skin bleaching agent which may produce undesired effects if not used as directed. The physician should be familiar with the contents of this insert before prescribing or dispensing this product.
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people.
Avoid unnecessary sun exposure, use an effective broad-spectrum sunscreen agent or protective clothing should be worn to cover bleached skin to prevent re-pigmentation from occurring.
Hydroquinone may produce exogenous ochronosis, a gradual blue-black darkening of the skin. If this condition occurs, discontinue treatment and consult your physician.
Avoid contact with eyes and mucous membranes. Keep out of reach of children. In case of accidental ingestion, call a physician or a poison control center immediately.
Precautions
Test for skin sensitivity before using by applying a small amount to an unbroken patch of skin; check within 24 hours. Minor redness is not a contraindication, but where there is itching or vesicle formation or excessive inflammatory response, further treatment is not advised. Close patient supervision is recommended.
Drug Interactions
Patients are cautioned on concomitant use of medications that are known to be photosensitizing.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies of hydroquinone in animals have demonstrated some evidence of carcinogenicity. The carcinogenic potential of hydroquinone in humans is unknown.
Pregnancy Category C
Animal reproduction studies have not been conducted with topical hydroquinone. It is also not known whether topical hydroquinone can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Topical hydroquinone should be given to a pregnant woman only if clearly needed.
Adverse Reactions/Side Effects
The following reactions have been reported: dryness and fissuring of paranasal and infraorbital areas, erythema, and stinging. Occasional hypersensitivity (localized contact dermatitis) may develop. If this occurs, the medication should be discontinued, and the physician notified immediately.
Related/similar drugs
Overdosage
There have been no system reactions reported from the use of topical hydroquinone. However, treatment should be limited to relatively small areas of the body at one time, since some patients experience a transient skin reddening and a mild burning sensation which does not preclude treatment.
Pigment Control Program - Hydroquinone Dosage and Administration
A thin layer of Pigment Control Creme (Hydroquinone USP, 4%) should be applied to the affected area twice daily or as directed by a physician. If no improvement is seen after 8-12 weeks of treatment, use of this product should be discontinued. There is no recommended dosage for pediatric patients under 12 years of age except under the advice and supervision of a physician.
How is Pigment Control Program - Hydroquinone supplied
Pigment Control Creme (Hydroquinone USP, 4%) is available as follows:
2.7 Fl. Oz. (80 mL) Bottle / NDC 42851-037-80
1.0 fl oz/30 ml Bottle / NDC 42851-037-30
PIGMENT CONTROL + BLENDING CREME
(Hydroquinone USP, 4%)
RX ONLY
FOR EXTERNAL USE ONLY:
NOT FOR OPHTHALMIC USE
Pigment Control Program - Hydroquinone Description
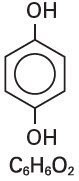
Hydroquinone is 1,4-benzendiol, with a chemical formula of C6H6O2 and a molecular weight of 110.11.
The structural formula is:
Each gram of Pigment Control + Blending Creme contains Hydroquinone USP 40mg/gm in a base of Ascorbic Acid, Ascorbyl Palmitate, Beta-Glucan, Caprylyl Glycol, Cetyl Alcohol, Chlorphenesin, Dioscorea Villosa (Wild Yam) Root Extract, Disodium EDTA, Ethylhexyl Palmitate, Glycerin, Glycolic Acid, Palmitic Acid, Phenoxyethanol, Phenyl Trimethicone, Purified Water, Quillaja Saponaria Bark Extract, Smilax Aristolochiifolia Root Extract, Sodium Hydroxide, Sodium Lauryl Sulfate, Sodium Metabisulfite, Sodium Sulfite, Stearyl Alcohol, Tocopheryl Acetate, Yucca Schidigera Root Extract.
Pigment Control Program - Hydroquinone - Clinical Pharmacology
Topical application of hydroquinone produces a reversible depigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to 3,4-dihydroxyphenylalanine (DOPA) and suppression of other melanocyte metabolic processes. Exposure to sunlight or ultraviolet light will cause re-pigmentation of bleached areas, which may be prevented by the use of the sunscreen agents.
Indications and Usage for Pigment Control Program - Hydroquinone
For the gradual bleaching of hyperpigmented skin conditions such as cholasma, melasma, freckles, senile lentigines, and other unwanted areas of melanin hyperpigmentation.
Contraindications
Prior history of sensitivity or allergic reaction to hydroquinone or to any other ingredient in this product. The safety of topical hydroquinone use during pregnancy or in children (12 years and under) has not been established.
Warnings
Hydroquinone is a skin bleaching agent which may produce undesired effects if not used as directed. The physician should be familiar with the contents of this insert before prescribing or dispensing this product.
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people.
Avoid unnecessary sun exposure, use an effective broad-spectrum sunscreen agent or protective clothing should be worn to cover bleached skin to prevent re-pigmentation from occurring.
Hydroquinone may produce exogenous ochronosis, a gradual blue-black darkening of the skin. If this condition occurs, discontinue treatment and consult your physician.
Avoid contact with eyes and mucous membranes. Keep out of reach of children. In case of accidental ingestion, call a physician or a poison control center immediately.
Precautions
Test for skin sensitivity before using by applying a small amount to an unbroken patch of skin; check within 24 hours. Minor redness is not a contraindication, but where there is itching or vesicle formation or excessive inflammatory response, further treatment is not advised. Close patient supervision is recommended.
Drug Interactions
Patients are cautioned on concomitant use of medications that are known to be photosensitizing.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies of hydroquinone in animals have demonstrated some evidence of carcinogenicity. The carcinogenic potential of hydroquinone in humans is unknown.
Pregnancy Category C
Animal reproduction studies have not been conducted with topical hydroquinone. It is also not known whether topical hydroquinone can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Topical hydroquinone should be given to a pregnant woman only if clearly needed.
Adverse Reactions/Side Effects
The following reactions have been reported: dryness and fissuring of paranasal and infraorbital areas, erythema, and stinging. Occasional hypersensitivity (localized contact dermatitis) may develop. If this occurs, the medication should be discontinued, and the physician notified immediately.
Overdosage
There have been no system reactions reported from the use of topical hydroquinone. However, treatment should be limited to relatively small areas of the body at one time, since some patients experience a transient skin reddening and a mild burning sensation which does not preclude treatment.
Pigment Control Program - Hydroquinone Dosage and Administration
A thin application of Pigment Control + Blending Creme should be applied to the affected area twice daily or as directed by a physician. Consult product label for instructions on whether to rub in or not. There is no recommendation for children under 12 years of age except under the advice and supervision of a physician.
How is Pigment Control Program - Hydroquinone supplied
Pigment Control + Blending Creme (Hydroquinone USP, 4%) is available as follows:
2.7 Fl. Oz. (80 mL) Bottle / NDC 42851-036-80
1.0 Fl. Oz (30 mL) Bottle / NDC 42851-036-30
PRINCIPAL DISPLAY PANEL - Kit Carton
ZO
®SKIN HEALTH
BY ZEIN OBAGI MD
PIGMENT CONTROL PROGRAM
+ HYDROQUINONE
NDC 42851-184-60
GENTLE CLEANSER 60 mL / 2 Fl. Oz.
EXFOLIATING POLISH Net Wt. 16.2 g / 0.57 Oz.
COMPLEXION RENEWAL PADS 30 Pads
PIGMENT CONTROL CRÈME 30 mL / 1.0 Fl. Oz.
DAILY POWER DEFENSE 30 mL / 1 Fl. Oz.
PIGMENT CONTROL + BLENDING CREME 30 mL / 1 Fl. Oz.

| ZO SKIN HEALTH PIGMENT CONTROL PROGRAM PLUS HYDROQUINONE
hydroquinone kit |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - ZO Skin Health, Inc. (826468527) |
More about hydroquinone topical
- Compare alternatives
- Pricing & coupons
- Reviews (8)
- Latest FDA alerts (1)
- Side effects
- Dosage information
- During pregnancy
- Drug class: topical depigmenting agents
- Breastfeeding
- En español
Patient resources
Professional resources
- Hydroquinone monograph
- Hydroquinone Cream (FDA)
- Hydroquinone Cream with Sunscreens (FDA)
- Hydroquinone Time Release Cream (FDA)
Other brands
Melamin, Lustra, Melquin HP, Glytone Skin Lightening, ... +4 more
