Lido-Rx Cream: Package Insert / Prescribing Info
Package insert / product label
Generic name: lidocaine, capsaicin
Dosage form: cream
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
On This Page
Lido-Rx Cream Description
Disclaimer: The FDA has not evaluated this drug, and FDA has not approved this labeling.
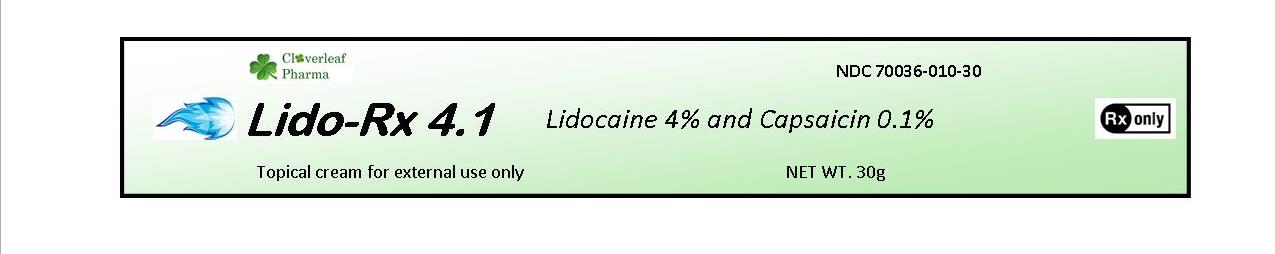
Lido-Rx 4.1 is a Lidocaine 4% and Capsaicin 0.1% cream which is a topical anesthetic and
analgesic indicated for the relief of pain related to minor cuts, grazes and irritation.
NOTE: There is no menthol in this product.
Indications and Usage for Lido-Rx Cream
Lido-Rx 4.1 is a topical anesthetic and analgesic indicated for the relief of pain related to minor cuts,
grazes and irritations.
Warnings
For external use only.
Use only as directed.
Avoid contact with eyes and mucous membranes.
Do not cover with bandage.
Do not use on wounds or damaged skin.
Consult physician for children under 12.
Do not use if you are allergic to Capsaicin or Lidocaine
Stop use and ask a doctor if conditions worsen, symptoms persist for more than 7 days or clear up and occur
again within a few days or rash, itching or excessive skin irritation occurs.
KEEP OUT OF REACH OF CHILDREN.
Precautions
If irritation or sensitivity occurs or infection appears, discontinue use and institute appropriate therapy.
Lido-Rx 4.1 Cream should be used with caution in ill, elderly, debilitated patients and children who may
be more sensitive to the systemic effects of lidocaine.
Carcinogenesis, Mutagenesis, and Impairment of Fertility:
Studies of lidocaine in animals to evaluate the carcinogenic and mutagenic potential of the effect on fertility
have not been conducted.
USE IN PREGNANCY:
Teratogenic Effects; Pregnancy Category B.
Reproduction studies have been performed for lidocaine in rats at doses up to 6.6 times the human dose and
have revealed no evidence of harm to the fetus caused by lidocaine. There are, however, no adequate and
well-controlled studies in pregnant women. Animal reproduction studies are not always predictive of human
response. General consideration should be given to this fact before administering lidocaine to women of
childbearing potential, especially during early pregnancy when maximum organogenesis takes place.
NURSING MOTHERS:
Lidocaine is excreted in human milk. The clinical significance of this observation is unknown.
Caution should be exercised when lidocaine is administered to a nursing woman.
PEDIATRIC USE:
Dosage in pediatric patients should be reduced commensurate with age, body weight, and physical condition.
ADVERSE REACTIONS:
During or immediately after treatment, the skin at the site of treatment may develop erythema or edema or
may be the locus of abnormal sensation.
CALL YOUR DOCTOR ABOUT SIDE EFFECTS.
Related/similar drugs
Lido-Rx Cream Dosage and Administration
Instructions for Use
Clean and dry affected area
Apply directly to area of pain
Apply to affected area not more than 4 times daily
Wash hands with soap after applying.
Adults and children 12 years and over
apply to affected area not more than 4 times daily
Children under 12 years consult physician before use
Call your doctor about side effects. You may report side effects to the FDA at 1-800-FDA-1088
1-800-FDA-1088 FREE.
KEEP THIS AND ALL MEDICATIONS OUT OF REACH OF CHILDREN.
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange
Book product. This product may be administered only under a physician's supervision.
There are no implied or explicit claims on the therapeutic equivalence.
Store at 25ºC (77ºF); excursions permitted to 15º-30ºC (59º-86º F). See USP Controlled Room Temperature.
Protect from freezing.
How is Lido-Rx Cream supplied
Lido-Rx 4.1 is supplied in a single box with a 5, 30 or 60 gram tube inside the box.
Lido-Rx 4.1 Cream 60 gram tube NDC 70036-010-60
Lido-Rx 4.1 Cream 30 gram tube NDC 70036-010-30
Lido-Rx 4.1 Cream 30 gram tube NDC 70036-010-05
Storage: Store below 25°C. Avoid direct sunlight.
Distributed by:
Cloverleaf Pharma, LLC, Fl 32502
770-579-8883
Manufactured exclusively for Cloverleaf Pharma LLC, by:
Pocono Coated Products, LLC
Greensboro, N.C. USA 27407
Purified Water, Isopropyl Palmitate, Propylene Glycol, Glyceryl Sterate, Isononyl Isonanoate, Glycerin, Lanolin Oil,
Myristyl Myristate, Stearic Acid, Carbmer, Methylparaben, Diazolidinyl Urea, Iodopropynyl Butylcarbamate,
Disodium EDTA, Allantoin, Triethanolamine, Sorbitan Sterate, Polysorbate, Dimethicone, Propylparaben,
BHA Tenox, Aloe Vera Gel, Vitamin A, Vitamin D, Tocopheryl Acetate
|
LIDO-RX
4.1
lidocaine 4.0% capsaicin 0.10% cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Cloverleaf Pharma LLC (079818984) |
| Registrant - Cloverleaf Pharma LLC (079818984) |