Isoxsuprine Hydrochloride: Package Insert / Prescribing Info
Package insert / product label
Dosage form: tablet
Drug class: Peripheral vasodilators
Medically reviewed by Drugs.com. Last updated on Jun 9, 2025.
On This Page
Isoxsuprine Hydrochloride Description
Each tablet taken orally contains Isoxsuprine Hydrochloride, USP with the following chemical structure:
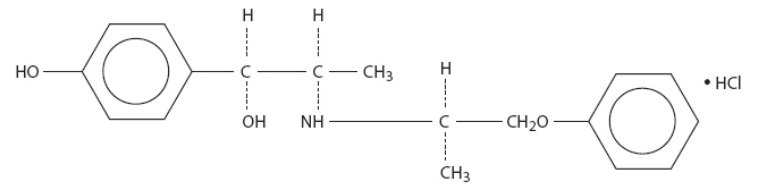
C18H23NO3 • HCl
p-Hydroxy-α[1-[(methyl-2-phenoxy-ethyl)amino]ethyl]benzyl alcohol hydrochloride.
Indications and Usage for Isoxsuprine Hydrochloride
Based on a review of this drug by the National Academy of Sciences-National Research and/or other information, the FDA has classified the indications as follows:
Possibly Effective:
- For the relief of symptoms associated with cerebrovascular insufficiency.
- In peripheral vascular disease of arteriosclerosis obliterans, thromboangitis obliterans (Buerger's disease) and Raynaud's disease.
Final classification of the less-than-effective indications requires further investigation.
Contraindications
There are no known contraindications to oral use when administered in recommended doses.
Isoxsuprine Hydrochloride, USP should not be given immediately postpartum or in the presence of arterial bleeding.
Adverse Reactions/Side Effects
On rare occasion oral administration of the drug has been associated in time with the occurrence of hypotension, tachycardia, chest pain, nausea, vomiting, dizziness, abdominal distress, and severe rash. If rash appears, the drug should be discontinued.
Although available evidence suggests a temporal association of these reactions with Isoxsuprine Hydrochloride, a causal relationship can be neither confirmed nor refuted.
Beta Adrenergic receptor stimulants such as Isoxsuprine Hydrocholoride have been used to inhibit pre-term labor.
Maternal and fetal tachycardia may occur under such use. Hypocalcemia, hypoglycemia, hypotension and ileus have been reported to occur in infants whose mothers received Isoxsuprine Hydrochloride. Pulmonary edema has been reported in mothers treated with beta stimulants. Isoxsuprine Hydrochloride is neither approved nor recommended for use in the treatment of premature labor.
Related/similar drugs
Isoxsuprine Hydrochloride Dosage and Administration
Oral: 10 to 20 mg, three or four times daily.For additional dosage recommendations, and other important prescribing information, see accompanying package insert.
How is Isoxsuprine Hydrochloride supplied
Isoxsuprine HCl tablets, USP 10 mg
Bottles of 100 NDC 42582-101-10
Isoxsuprine HCl tablets, USP 20 mg
Bottles of 100 NDC 42582-201-10
Isoxsuprine HCl tablets, USP 20 mg
Bottles of 1000 NDC 42582-201-20
COMPOSITION
lsoxsuprine HCI 10mg and 20mg tablets:
These tablets contain the fdlowing inactive ingredients: lactose monohydrate, magnesium stearate, microcrystalline cellulose.
| ISOXSUPRINE HYDROCHLORIDE
isoxsuprine hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Syntho Pharmaceuticals Inc. (088797407) |
| Registrant - Syntho Pharmaceuticals Inc. (088797407) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Syntho Pharmaceuticals Inc. | 088797407 | manufacture(66576-201) | |
More about isoxsuprine
- Check interactions
- Compare alternatives
- Reviews (3)
- Drug images
- Side effects
- Drug class: peripheral vasodilators