Glucovance: Package Insert / Prescribing Info
Package insert / product label
Generic name: glyburide and metformin hydrochloride
Dosage form: tablet, film coated
Drug class: Antidiabetic combinations
Medically reviewed by Drugs.com. Last updated on Jun 16, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
Initial U.S. Approval: 2000
WARNING: LACTIC ACIDOSIS
See full prescribing information for complete boxed warning.
- Post-marketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. Symptoms include malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Laboratory abnormalities included elevated blood lactate levels, anion gap acidosis, increased lactate/pyruvate ratio; and metformin plasma levels generally >5 mcg/mL. (5.1)
- Risk factors include renal impairment, concomitant use of certain drugs, age ≥65 years old, radiological study with contrast, surgery and other procedures, hypoxic states, excessive alcohol intake, and hepatic impairment. Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided in the Full Prescribing Information. (5.1).
- If lactic acidosis is suspected, discontinue GLUCOVANCE and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended. (5.1)
Indications and Usage for Glucovance
GLUCOVANCE is a combination of glyburide, a sulfonylurea, and metformin hydrochloride (HCl), a biguanide, indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. (1)
Glucovance Dosage and Administration
Adult Dosage:
- Give GLUCOVANCE in divided doses, twice daily, with meals. (2.1)
- For patients not treated with either glyburide (or another sulfonylurea) or metformin HCl, initiate treatment with another formulation with a dose of 1.25 mg glyburide and 250mg metformin HCl orally, once or twice daily with meals. (2.1)
- For patients not adequately controlled on either glyburide (or another sulfonylurea) or metformin HCl alone, the recommended starting dose is 2.5 mg/500 mg or 5 mg/500 mg orally twice daily with meals. (2.1)
- For patients previously treated with a combination therapy of glyburide (or another sulfonylurea) and metformin HCl, the starting dose should not exceed the daily dose of glyburide (or equivalent dose of another sulfonylurea) and metformin HCl already being taken. (2.1)
- Increase the dose gradually on the basis of glycemic control and • tolerability, up to a maximum to a maximum dose of 20 mg glyburide/2000 mg metformin HCl daily. (2.1)
Renal Impairment:
Prior to initiation, assess renal function with estimated glomerular filtration rate (eGFR) (2.4)
o Do not use in patients with eGFR below 30 mL/minute/1.73 m2 (2.4)
o Initiation is not recommended in patients with eGFR between 30 to 45 mL/minute/1.73 m2 (2.4)
o Assess risk/benefit if eGFR falls below 45 mL/minute/1.73 m2 (2.4)
o Discontinue if eGFR falls below 30 mL/minute/1.73 m2 (2.4)
Discontinuation for Iodinated Contrast Imaging Procedures:
- GLUCOVANCE may need to be discontinued at time of, or prior to, iodinated contrast imaging procedures (2.5)
Dosage Forms and Strengths
Contraindications
Warnings and Precautions
- Lactic Acidosis: See boxed warning. (5.1)
- Hypoglycemia: May be severe. Ensure proper patient selection, dosing, and instructions, particularly in at-risk populations (e.g., elderly, renally impaired) and when used with other anti-diabetic medications. (5.2)
- Potential Increased Risk of Cardiovascular Mortality with Sulfonylureas: Inform patient of risks, benefits and treatment alternatives. (5.3)
- Hemolytic anemia: Can occur if glucose 6-phosphate dehydrogenase (G6PD) deficient. Consider a non-sulfonylurea alternative. (5.4)
- Vitamin B12 Deficiency: Metformin may lower vitamin B12 levels. Measure hematological parameters annually and vitamin B12 at 2 to 3 year intervals and manage any abnormalities. (5.5)
Adverse Reactions/Side Effects
Most common (>5%) adverse reactions to GLUCOVANCE diarrhea, headache, nausea/vomiting, abdominal pain, and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bristol-Myers Squibb at 1-800-721-5072 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Carbonic anhydrase inhibitors may increase risk of lactic acidosis. Consider more frequent monitoring. (7)
- Drugs that reduce metformin clearance (such as ranolazine, vandetanib, dolutegravir, and cimetidine) may increase the accumulation of metformin. Consider the benefits and risks of concomitant use. (7)
- Alcohol can potentiate the effect of metformin on lactate metabolism. Warn patients against excessive alcohol intake. (7)
- The hypoglycemic action of GLUCOVANCE may be potentiated by certain drugs. (7)
- Concomitant administration of colesevalam may led to reduced glyburide absorption. (7)
Use In Specific Populations
- Pregnancy: GLUCOVANCE should be discontinued at least two weeks before expected delivery. (8.1)
- Females and Males of Reproductive Potential: Advise premenopausal females of the potential for an unintended pregnancy. (8.3)
- Geriatric Use: Assess renal function more frequently. (8.5)
- Hepatic Impairment: Avoid use in patients with hepatic impairment. (8.7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2018
Full Prescribing Information
WARNING: LACTIC ACIDOSIS
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin-associated lactic acidosis was characterized by elevated blood lactate levels (>5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally >5 mcg/mL [see Warnings and Precautions (5.1)].
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g., carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided [see Dosage and Administration (2.3), Contraindications (4) and Warnings and Precautions (5.1)].
If metformin-associated lactic acidosis is suspected, immediately discontinue GLUCOVANCE and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended [see Warnings and Precautions (5.1)].
1. Indications and Usage for Glucovance
GLUCOVANCE is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
2. Glucovance Dosage and Administration
2.1 Dosage
- Give GLUCOVANCE in divided doses, twice daily, with meals.
- For patients not treated with either glyburide (or another sulfonylurea) or metformin hydrochloride (HCl), initiate treatment with another formulation of glyburide and metformin HCl at a starting dose of 1.25 mg glyburide and 250mg metformin HCl orally, once or twice daily with meals.
- For patients not adequately controlled on either glyburide (or another sulfonylurea) or metformin HCl alone, the recommended starting dose of GLUCOVANCE is 2.5 mg/500 mg or 5 mg/500 mg orally twice daily with meals.
- For patients previously treated with a combination therapy of glyburide (or another sulfonylurea) and metformin HCl, the starting dose of GLUCOVANCE should not exceed the daily dose of glyburide (or equivalent dose of another sulfonylurea) and metformin HCl already being taken.
- • Increase the dose gradually on the basis of glycemic control and tolerability, up to a maximum to a maximum dose of 20 mg glyburide/2000 mg metformin HCl daily.
2.2 Patients Receiving Colesevelam
- Administer GLUCOVANCE at least 4 hours prior to colesevelam for patients taking both drugs concomitantly [see Drug Interactions (7)].
2.3 Recommendations for Use in Renal Impairment
- Assess renal function prior to initiation of GLUCOVANCE and periodically thereafter.
- GLUCOVANCE is contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/minute/1.73 m2.
- Initiation of GLUCOVANCE in patients with an eGFR between 30 to 45 mL/minute/1.73 m2 is not recommended.
- In patients taking GLUCOVANCE whose eGFR later falls below 45 mL/min/1.73 m2, assess the benefit risk of continuing therapy.
- Discontinue GLUCOVANCE if the patient’s eGFR later falls below 30 mL/minute/1.73 m2 [see Warnings and Precautions (5.1)].
2.4 Discontinuation for Iodinated Contrast Imaging Procedures
- Discontinue GLUCOVANCE at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of liver disease, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast.
- Re-evaluate eGFR 48 hours after the imaging procedure; restart GLUCOVANCE if renal function is stable.
3. Dosage Forms and Strengths
GLUCOVANCE is available as:
- Tablets: glyburide 2.5 mg and metformin HCl 500 mg pale orange, capsule-shaped, bevel-edged, biconvex, film-coated tablet with "BMS" debossed on one side and "6073" debossed on the opposite side.
- Tablets: glyburide 5 mg and metformin HCl 500 mg yellow, capsule-shaped, bevel-edged, biconvex, film-coated tablet with "BMS" debossed on one side and "6074" debossed on the opposite side.
4. Contraindications
GLUCOVANCE is contraindicated in patients with:
- Severe renal impairment (eGFR below 30 mL/min/1.73 m2) [see Warnings and Precautions (5.1)].
- Hypersensitivity to metformin or glyburide.
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis, with or without coma.
- Concomitant administration of bosentan [see Drug Interactions (7)].
5. Warnings and Precautions
5.1 Lactic Acidosis
There have been postmarketing cases of metformin-associated lactic acidosis, including fatal cases. These cases had a subtle onset and were accompanied by nonspecific symptoms such as malaise, myalgias, abdominal pain, respiratory distress, or increased somnolence; however, hypotension and resistant bradyarrhythmias have occurred with severe acidosis. Metformin-associated lactic acidosis was characterized by elevated blood lactate concentrations (>5 mmol/L), anion gap acidosis (without evidence of ketonuria or ketonemia), and an increased lactate: pyruvate ratio; metformin plasma levels were generally >5 mcg/mL. Metformin decreases liver uptake of lactate increasing lactate blood levels which may increase the risk of lactic acidosis, especially in patients at risk.
If metformin-associated lactic acidosis is suspected, general supportive measures should be instituted promptly in a hospital setting, along with immediate discontinuation of GLUCOVANCE. In GLUCOVANCE treated patients with a diagnosis or strong suspicion of lactic acidosis, prompt hemodialysis is recommended to correct the acidosis and remove accumulated metformin (metformin HCl is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions). Hemodialysis has often resulted in reversal of symptoms and recovery.
Educate patients and their families about the symptoms of lactic acidosis and, if these symptoms occur, instruct them to discontinue GLUCOVANCE and report these symptoms to their healthcare provider.
For each of the known and possible risk factors for metformin-associated lactic acidosis, recommendations to reduce the risk of and manage metformin-associated lactic acidosis are provided below:
- Renal Impairment—The postmarketing metformin-associated lactic acidosis cases primarily occurred in patients with significant renal impairment.The risk of metformin accumulation and metformin-associated lactic acidosis increases with the severity of renal impairment because metformin is substantially excreted by the kidney. Clinical recommendations based upon the patient’s renal function include [see Dosage and Administration (2.1), Clinical Pharmacology (12.3)]:
- Before initiating GLUCOVANCE, obtain an estimated glomerular filtration rate (eGFR).
- GLUCOVANCE is contraindicated in patients with an eGFR less than 30 mL/min/1.73 m2 [see CONTRAINDICATIONS (4)].
- Initiation of GLUCOVANCE is not recommended in patients with eGFR between 30 to 45 mL/min/1.73 m2.
- Obtain an eGFR at least annually in all patient taking GLUCOVANCE. In patients at risk for the development of renal impairment (e.g., the elderly), renal function should be assessed more frequently.
- In patients taking GLUCOVANCE whose eGFR falls below 45 mL/min/1.73 m2, assess the benefit and risk of continuing therapy.
- Drug interactions—The concomitant use of GLUCOVANCE with specific drugs may increase the risk of metformin-associated lactic acidosis: those that impair renal function, result in significant hemodynamic change, interfere with acid-base balance, or increase metformin accumulation [see Drug Interactions (7)]. Consider more frequent monitoring of patients.
- Age 65 or Greater—The risk of metformin-associated lactic acidosis increases with the patient’s age because elderly patients have a greater likelihood of having hepatic, renal, or cardiac impairment than younger patients. Assess renal function more frequently in elderly patients.
- Radiologic studies with contrast—Administration of intravascular iodinated contrast agents in metformin-treated patients has led to an acute decrease in renal function and the occurrence of lactic acidosis. Stop GLUCOVANCE at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism or heart failure, or in patients who will be administered intra-arterial iodinated contrast. Reevaluate eGFR 48 hours after the imaging procedure, and restart GLUCOVANCE if renal function is stable.
- Surgery and other procedures—Withholding of food and fluids during surgical or other procedures may increase the risk for volume depletion, hypotension, and renal impairment. GLUCOVANCE should be temporarily discontinued while patients have restricted food and fluid intake.
- Hypoxic states—Several of the postmarketing cases of metformin-associated lactic acidosis occurred in the setting of acute congestive heart failure (particularly when accompanied by hypoperfusion and hypoxemia). Cardiovascular collapse (shock), acute myocardial infarction, sepsis, and other conditions associated with hypoxemia have been associated with lactic acidosis and may cause prerenal azotemia. When such an event occurs, discontinue GLUCOVANCE.
- Excessive Alcohol intake—Alcohol potentiates the effect of metformin on lactate metabolism. Patients should be warned against excessive alcohol intake, acute or chronic, while receiving GLUCOVANCE.
- Hepatic impairment—Patients with hepatic impairment have developed cases of metformin-associated lactic acidosis. This may be due to impaired lactate clearance resulting in higher lactate blood levels. Therefore, avoid use of GLUCOVANCE in patients with clinical or laboratory evidence of hepatic disease.
5.2 Hypoglycemia
All sulfonylurea drugs, including GLUCOVANCE, are capable of producing severe hypoglycemia [see Adverse Reactions (6)]. Concomitant use of GLUCOVANCE with other anti-diabetic medication can increase the risk of hypoglycemia. A lower dose of GLUCOVANCE may be required to minimize the risk of hypoglycemia when combining it with other anti-diabetic medications.
Educate patients to recognize and manage hypoglycemia. When initiating and increasing GLUCOVANCE in patients who may be predisposed to hypoglycemia (e.g., the elderly, patients with renal impairment, patients on other anti-diabetic medications) start with a lower dose. Debilitated or malnourished patients, and those with adrenal, pituitary, or hepatic impairment are particularly susceptible to the hypoglycemic action of anti-diabetic medications. Hypoglycemia is also more likely to occur when caloric intake is deficient, after severe or prolonged exercise, or when alcohol is ingested.
The patient's ability to concentrate and react may be impaired as a result of hypoglycemia. Early warning symptoms of hypoglycemia may be different or less pronounced in patients with autonomic neuropathy, the elderly, and in patients who are taking beta-adrenergic blocking medications or other sympatholytic agents. These situations may result in severe hypoglycemia before the patient is aware of the hypoglycemia.
These impairments may present a risk in situations where these abilities are especially important, such as driving or operating other machinery. Severe hypoglycemia can lead to unconsciousness or convulsions and may result in temporary or permanent impairment of brain function or death.
5.3 Cardiovascular Mortality
The administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term prospective clinical study designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with type 2 diabetes mellitus. The study involved 823 patients who were randomly assigned to 1 of 4 treatment groups.
UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5 grams per day) had a rate of cardiovascular mortality approximately 2½ times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and benefits of glyburide and of alternative modes of therapy.
Although only one drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure.
5.4 Hemolytic Anemia
Treatment of patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency with sulfonylurea agents, including GLUCOVANCE, can lead to hemolytic anemia. Avoid use of GLUCOVANCE in patients with G6PD deficiency. In postmarketing reports, hemolytic anemia has also been reported in patients who did not have known G6PD deficiency.
5.5 Vitamin B12 Deficiency
In clinical studies of 29-week duration with metformin HCl tablets, a decrease to subnormal levels of previously normal serum vitamin B12 levels, was observed in approximately 7% of patients. Such decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex, may be associated with anemia but appears to be rapidly reversible with discontinuation of metformin or vitamin B12 supplementation. Certain individuals (those with inadequate vitamin B12 or calcium intake or absorption) appear to be predisposed to developing subnormal vitamin B12 levels. Measure hematologic parameters on an annual basis and vitamin B12 at 2 to 3 year intervals in patients on GLUCOVANCE and manage any abnormalities [see Adverse Reactions (6.1)].
6. Adverse Reactions/Side Effects
The following adverse reactions are also discussed elsewhere in the labeling:
- Lactic Acidosis [see Boxed Warning and Warnings and Precautions (5.1)]
- Hypoglycemia [see Warnings and Precautions (5.2)]
- Cardiovascular mortality [see Warnings and Precautions (5.3)]
- Hemolytic anemia [see Warnings and Precautions (5.4)]
- Vitamin B12 Deficiency [see Warnings and Precautions (5.5)]
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In double-blind clinical studies with GLUCOVANCE as initial therapy or as second-line therapy of 20 and 14 weeks, respectively (see section 14), a total of 642 patients received GLUCOVANCE, 312 received metformin HCl, 324 received glyburide, and 161 received placebo. Adverse reactions are listed in Table 1.
| Table 1: Adverse Reactions Occurring >5% in Double-Blind Clinical Studies of GLUCOVANCE Used as Initial (20 Weeks) or Second-Line (14 Weeks) Therapy | ||||
| Number (%) of Patients | ||||
|
Adverse Reaction Placebo Glyburide Metformin HCl GLUCOVANCE N=161 N=324 N=312 N=642 | ||||
| Diarrhea 6 % 6% 21% 17% | ||||
| Headache 11% 11% 9% 9% | ||||
|
Nausea/vomiting 6% 5% 12% 8% | ||||
|
Abdominal pain 4% 3% 8% 7% | ||||
| Dizziness 4% 6% 4% 6% |
Hypoglycemia
The incidence of reported symptoms of hypoglycemia (such as dizziness, shakiness, sweating, and hunger), in the initial therapy study of GLUCOVANCE are summarized in Table 2. For patients with a baseline HbA1c between 8% and 11% treated with GLUCOVANCE 2.5 mg/500 mg as initial therapy, the frequency of hypoglycemic symptoms was 30% to 35%. As second-line therapy in patients inadequately controlled on sulfonylurea alone, approximately 6.8% of all patients treated with GLUCOVANCE experienced hypoglycemic symptoms.
Gastrointestinal Reactions
The incidence of gastrointestinal (GI) side effects (diarrhea, nausea/vomiting, and abdominal pain) in the GLUCOVANCE initial therapy study are summarized in Table 2. Across all GLUCOVANCE studies, GI symptoms were the most common adverse events with GLUCOVANCE and were more frequent at higher dose levels. In controlled studies, <2% of patients discontinued GLUCOVANCE therapy due to GI adverse events.
| Table 2: Hypoglycemia or Gastrointestinal Adverse Reactions in a Placebo- and Active-Controlled Study of GLUCOVANCE as Initial Therapy (20 Weeks) | |||||
| Variable |
Placebo N=161 |
Glyburide Tablets N=160 |
Metformin HCl Tablets N=159 |
GLUCOVANCE 1.25 mg/250 mg N=158 |
GLUCOVANCE 2.5 mg/500 mg N=162 |
|
Number (%) of patients with symptoms of hypoglycemia | 3% | 21% | 3% | 11% |
38% |
|
Number (%) of patients with gastrointestinal adverse events | 24% | 24% | 43% |
32% |
38% |
Dermatologic Reactions
Allergic skin reactions, e.g., pruritus, erythema, urticaria, and morbilliform or maculopapular eruptions, occur in 1.5% of glyburide-treated patients. These may be transient and may disappear despite continued use.
6.2 Postmarketing Adverse Reactions
The following adverse reactions have been identified during post-approval use of GLUCOVANCE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Allergic: Angioedema, arthralgia, myalgia, and vasculitis have been reported.
Dermatologic: Porphyria cutanea tarda and photosensitivity reactions have been reported with sulfonylureas.
Hematologic: Leukopenia, agranulocytosis, thrombocytopenia, which occasionally may present as purpura, hemolytic anemia, aplastic anemia, and pancytopenia, have been reported with sulfonylureas.
Hepatic: Cholestatic, hepatocellular, and mixed hepatocellular liver injury have been reported with postmarketing use of metformin.
Cholestatic jaundice and hepatitis may occur rarely with glyburide, which may progress to liver failure. Liver function abnormalities, including isolated transaminase elevations, have been reported.
Metabolic: Hepatic porphyria reactions have been reported with sulfonylureas; however, these have not been reported with glyburide. Disulfiram-like reactions have been reported very rarely with glyburide. Cases of hyponatremia have been reported with glyburide and all other sulfonylureas, most often in patients who are on other medications or have medical conditions known to cause hyponatremia or increase release of antidiuretic hormone.
Other Reactions: Changes in accommodation and/or blurred vision have been reported with glyburide and other sulfonylureas. These are thought to be related to fluctuation in glucose levels.
Related/similar drugs
7. Drug Interactions
Table 3 presents clinically significant drug interactions with GLUCOVANCE.
| Table 3: Clinically Significant Drug Interactions with Glucovance | |
| Carbonic Anhydrase Inhibitors | |
| Clinical Impact: | Carbonic anhydrase inhibitors frequently cause a decrease in serum bicarbonate and induce non-anion gap, hyperchloremic metabolic acidosis. Concomitant use of these drugs with GLUCOVANCE may increase the risk for lactic acidosis. |
| Intervention: | Consider more frequent monitoring of these patients. |
| Examples: | Topiramate, zonisamide, acetazolamide and dichlorphenamide. |
| Drugs that Reduce Metformin Clearance | |
| Clinical Impact: | Concomitant use of drugs that interfere with common renal tubular transport systems involved in the renal elimination of metformin (e.g., organic cationic transporter-2 [OCT2] / multidrug and toxin extrusion [MATE] inhibitors) could increase systemic exposure to metformin and may increase the risk for lactic acidosis [see Clinical Pharmacology (12.3)]. |
| Intervention: | Consider the benefits and risks of concomitant use with GLUCOVANCE. |
| Examples: | Ranolazine, vandetanib, dolutegravir, and cimetidine. |
| Alcohol | |
| Clinical Impact: | Alcohol is known to potentiate the effect of metformin on lactate metabolism. |
| Intervention: | Warn patients against excessive alcohol intake while receiving GLUCOVANCE. |
| Drugs that potentiate the hypoglycemic action of GLUCOVANCE | |
| Clinical Impact: | Certain drugs may potentiate the hypoglycemic action of sulfonylureas, one of the components of GLUCOVANCE. |
| Intervention: | Closely observe patient for hypoglycemia during co-administration and for loss of glycemic control when withdrawing these agents. |
| Examples: | Nonsteroidal anti-inflammatory agents and other highly protein-boind drugs, salicylcates, sulfonamides, chloramphenicol, probenecid, coumarins, monoamine oxidase inhibitors, beta-adrenergic blocking agents; potentially with ciprofloxacin, micronazole. |
| Bosentan | |
| Clinical Impact: | Increased risk of liver enzyme elevations was observed. |
| Intervention: | Concomitant administration is contraindicated. |
| Colesevalam | |
| Clinical Impact: | Concomitant administration may led to reduced glyburide absorption (AUC and Cmax: -32% and -47%, respectively). |
| Intervention: | GLUCOVANCE should be administered at least 4 hours prior to colesevelam. |
| Drugs Reducing Glycemic Control | |
| Clinical Impact: | Certain drugs tend to produce hyperglycemia and may lead to loss of glycemic control. |
| Intervention: | When such drugs are administered to a patient receiving GLUCOVANCE observe the patient closely for loss of blood glucose control. When such drugs are withdrawn from a patient receiving GLUCOVANCE, observe the patient closely for hypoglycemia. |
| Examples: | Thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blockers, and isoniazid. |
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Available data from a small number of published studies and postmarketing experience with glyburide use in pregnancy over decades have not identified any drug associated risks for major birth defects, miscarriage, or adverse maternal outcomes. However, sulfonylureas (including glyburide) cross the placenta and have been associated with neonatal adverse reactions such as hypoglycemia. Therefore, GLUCOVANCE should be discontinued at least two weeks before expected delivery [see Clinical Considerations]. Limited data with metformin in pregnant women are not sufficient to determine a drug-associated risk for major birth defects or miscarriage. Published studies with metformin use during pregnancy have not reported a clear association with metformin and major birth defect or miscarriage risk [see Data]. There are risks to the mother and fetus associated with poorly controlled diabetes mellitus in pregnancy [see Clinical Considerations].
No evidence of harm to the fetus was observed when doses up to 500 times the maximum recommended human dose of 20 mg of glyburide, based on body surface area, were administered to rats and rabbits in reproduction studies.
No adverse developmental effects were observed when metformin was administered to pregnant Sprague Dawley rats and rabbits during the period of organogenesis at doses up to 3- and 6-times, respectively, a 2000 mg clinical dose, based on body surface area [see Data].
The estimated background risk of major birth defects is 6–10% in women with pre-gestational diabetes mellitus with an HbA1c >7 and has been reported to be as high as 20–25% in women with a HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Poorly controlled diabetes mellitus in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, and delivery complications. Poorly controlled diabetes mellitus increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Fetal/Neonatal Adverse Reactions
Neonates of women with gestational diabetes who are treated with sulfonylureas during pregnancy may be at increased risk for neonatal intensive care admission and may develop respiratory distress, hypoglycemia, birth injury, and be large for gestational age. Prolonged severe hypoglycemia, lasting 4-10 days, has been reported in neonates born to mothers receiving a sulfonylurea at the time of delivery and has been reported with the use of agents with a prolonged half-life. Observe newborns for symptoms of hypoglycemia and respiratory distress and manage accordingly.
Dose adjustments during pregnancy and the postpartum period
Due to reports of prolonged severe hypoglycemia in neonates born to mothers receiving a sulfonylurea at the time of delivery, GLUCOVANCE should be discontinued at least two weeks before expected delivery [see Fetal/Neonatal Adverse Reactions].
Data
Human Data
Published data from post-marketing studies have not reported a clear association with metformin and major birth defects, miscarriage, or adverse maternal or fetal outcomes when metformin was used during pregnancy. However, these studies cannot definitely establish the absence of any metformin-associated risk because of methodological limitations, including small sample size and inconsistent comparator groups.
Animal Data
Reproduction studies were performed in rats and rabbits at doses up to 500 times the maximum recommended human dose of 20 mg of glyburide based on body surface area comparisons and revealed no evidence of harm to the fetus.
Metformin did not adversely affect development outcomes when administered to pregnant rats and rabbits at doses up to 600 mg/kg/day. This represents an exposure of about 3 and 6 times a 2000 mg clinical dose based on body surface area comparisons for rats and rabbits, respectively. Determination of fetal concentrations demonstrated a partial placental barrier to metformin.
8.2 Lactation
Risk Summary
Breastfed infants of lactating women using GLUCOVANCE should be monitored for symptoms of hypoglycemia [see Clinical Considerations]. Although glyburide was negligible in human milk in one small clinical lactation study; this result is not conclusive because of the limitations of the assay used in the study. There are no data on the effects of glyburide on milk production. Limited published studies report that metformin is present in human milk [see Data]. However, there is insufficient information to determine the effects of metformin on the breastfed infant and no available information on the effects of metformin on milk production. Therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for GLUCOVANCE and any potential adverse effects on the breastfed child from GLUCOVANCE or from the underlying maternal condition.
Clinical Considerations
Monitoring for adverse reactions
Monitor breastfed infants for signs of hypoglycemia (e.g., jitters, cyanosis, apnea, hypothermia, excessive sleepiness, poor feeding, seizures).
Data
Published clinical lactation studies report that metformin is present in human milk which resulted in infant doses approximately 0.11% to 1% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 0.13 and 1. However, the studies were not designed to definitely establish the risk of use of metformin during lactation because of small sample size and limited adverse event data collected in infants.
8.3 Females and Males of Reproductive Potential
Discuss the potential for unintended pregnancy with premenopausal women as therapy with GLUCOVANCE may result in ovulation in some anovulatory women.
8.4 Pediatric Use
Safety and effectiveness of GLUCOVANCE have not been established in pediatric patients.
8.5 Geriatric Use
Of the 642 patients who received GLUCOVANCE in double-blind clinical studies, 23.8% were 65 and older while 2.8% were 75 and older. No overall differences in effectiveness or safety were observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Elderly patients are particularly susceptible to the hypoglycemic action of anti-diabetic agents. Hypoglycemia may be difficult to recognize in these patients.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy and the higher risk of hypoglycemia and lactic acidosis. Assess renal function more frequently in elderly patients [see Dosage and Administration (2) and Warnings and Precautions (5.1)].
8.6 Renal Impairment
Metformin is substantially excreted by the kidney, and the risk of metformin accumulation and lactic acidosis increases with the degree of renal impairment.
GLUCOVANCE is contraindicated in severe renal impairment, patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2 [see Dosage and Administration (2.3), Contraindications (4), Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Use of metformin in patients with hepatic impairment has been associated with some cases of lactic acidosis. GLUCOVANCE is not recommended in patients with hepatic impairment [see Warnings and Precautions (5.1)].
10. Overdosage
Glyburide
Overdosage of sulfonylureas, including glyburide tablets, can produce hypoglycemia. Mild hypoglycemic symptoms, without loss of consciousness or neurological findings, should be treated with oral glucose. Severe hypoglycemic reactions with coma, seizure, or other neurological impairment are medical emergencies requiring immediate treatment. The patient should be treated with glucagon or intravenous glucose. Patients should be closely monitored for a minimum of 24 to 48 hours since hypoglycemia may recur after apparent clinical recovery. Clearance of glyburide from plasma may be prolonged in persons with liver disease. Because of the extensive protein binding of glyburide, dialysis is unlikely to be of benefit.
Metformin
Overdose of metformin has occurred, including ingestion of amounts greater than 50 g. Hypoglycemia was reported in approximately 10% of cases, but no causal association with metformin has been established. Lactic acidosis has been reported in approximately 32% of metformin overdose cases [see Warnings and Precautions (5.1)]. Metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful for removal of accumulated drug from patients in whom metformin overdosage is suspected.
11. Glucovance Description
GLUCOVANCE tablets for oral use contain glyburide and metformin hydrochloride.
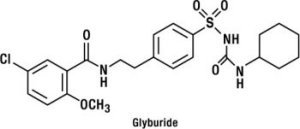
Glyburide is a sulfonylurea and its chemical name is 1-[[p-[2-(5-chloro-o-anisamido) ethyl]phenyl]sulfonyl]-3-cyclo-hexylurea. Glyburide is a white to off-white crystalline compound with a molecular formula of C23H28ClN3O5S and a molecular weight of 494.01. The glyburide used in GLUCOVANCE has a particle size distribution of 25% undersize value not more than 6 µm, 50% undersize value not more than 7 to 10 µm, and 75% undersize value not more than 21 µm. The structural formula is represented below.
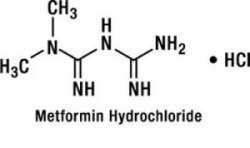
Metformin hydrochloride is a biguanide in hydrochloride salt form and its chemical name is N,N-dimethylimidodicarbonimidic diamide monohydrochloride. It is a white to off-white crystalline compound with a molecular formula of C4H12ClN5 (monohydrochloride) and a molecular weight of 165.63. Metformin is freely soluble in water and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin is 6.68. The structural formula is as shown:
GLUCOVANCE is available in film coated tablets containing:
- 2.5 mg glyburide and 500 mg metformin hydrochloride (equivalent to 389.93 mg metformin);
- 5 mg glyburide and 500 mg metformin hydrochloride (equivalent to 389.93 mg metformin).
Each tablet contains the inactive ingredients: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and povidone.
12. Glucovance - Clinical Pharmacology
12.1 Mechanism of Action
Glyburide primarily lowers blood glucose by stimulating the release of insulin from the pancreas, an effect dependent upon functioning beta cells in the pancreatic islets. Sulfonylureas bind to the sulfonylurea receptor in the pancreatic beta-cell plasma membrane, leading to closure of the ATP-sensitive potassium channel, thereby stimulating the release of insulin.
Metformin is an antihyperglycemic agent that improves glucose tolerance in patients with type 2 diabetes, lowering both basal and postprandial plasma glucose. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and daylong plasma insulin response may decrease.
12.3 Pharmacokinetics
Absorption
GLUCOVANCE
In bioavailability studies of GLUCOVANCE 2.5 mg/500 mg and 5 mg/500 mg, the mean area under the plasma concentration versus time curve (AUC) for the glyburide component was 18% and 7%, respectively, greater than that of standard particle-size glyburide coadministered with metformin. The pharmacokinetics of metformin HCl component of GLUCOVANCE was consistent with that of metformin HCl coadministered with glyburide.
Effect of food: Following administration of a single GLUCOVANCE 5 mg/500 mg tablet with either a 20% glucose solution or a 20% glucose solution with food, there was no effect of food on the Cmax and a relatively small effect of food on the AUC of the glyburide component. The Tmax for the glyburide component was shortened from 7.5 hours to 2.75 hours with food compared to the same tablet strength administered fasting with a 20% glucose solution. The effect of food on the pharmacokinetics of the metformin component of GLUCOVANCE was indeterminate. However, food is known to decrease the extent of and slightly delay the absorption of metformin, as shown by approximately a 40% lower mean peak plasma concentration (Cmax), a 25% lower area under the plasma concentration versus time curve (AUC), and a 35-minute prolongation of time to peak plasma concentration (Tmax) following administration of a single 850 mg tablet of metformin with food, compared to the same tablet strength administered fasting. The clinical relevance of these decreases is unknown.
Glyburide
Single-dose studies with standard particle-size glyburide tablets in normal subjects demonstrate significant absorption of glyburide within 1 hour, peak drug levels at about 4 hours, and low but detectable levels at 24 hours. Mean serum levels of glyburide, as reflected by areas under the serum concentration-time curve, increase in proportion to corresponding increases in dose. Bioequivalence has not been established between GLUCOVANCE and single-ingredient standard particle-size glyburide products.
Metformin
The absolute bioavailability of a 500 mg metformin tablet given under fasting conditions is approximately 50% to 60%. Studies using single oral doses of metformin tablets of 500 mg and 1500 mg, and 850 mg to 2550 mg, indicate that there is a lack of dose proportionality with increasing doses, which is due to decreased absorption rather than an alteration in elimination. At usual clinical doses and dosing schedules of metformin, steady-state plasma concentrations of metformin are reached within 24 to 48 hours and are generally <1 µg/mL.
Distribution
Glyburide
Sulfonylurea drugs are extensively bound to serum proteins. Displacement from protein binding sites by other drugs may lead to enhanced hypoglycemic action. In vitro, the protein binding exhibited by glyburide is predominantly non-ionic, whereas that of other sulfonylureas (chlorpropamide, tolbutamide, tolazamide) is predominantly ionic. Acidic drugs, such as phenylbutazone, warfarin, and salicylates, displace the ionic-binding sulfonylureas from serum proteins to a far greater extent than the non-ionic binding glyburide. It has not been shown that this difference in protein binding results in fewer drug-drug interactions with glyburide tablets in clinical use.
Metformin
The apparent volume of distribution (V/F) of metformin following single oral doses of 850 mg averaged 654±358 L. Metformin is negligibly bound to plasma proteins. Metformin partitions into erythrocytes, most likely as a function of time.
Metabolism and Elimination
Glyburide
The decrease of glyburide in the serum of normal healthy individuals is biphasic; the terminal half-life is about 10 hours. The major metabolite of glyburide is the 4-trans-hydroxy derivative. A second metabolite, the 3-cis-hydroxy derivative, also occurs. These metabolites probably contribute no significant hypoglycemic action in humans since they are only weakly active (1/400 and 1/40 as active, respectively, as glyburide) in rabbits. Glyburide is excreted as metabolites in the bile and urine, approximately 50% by each route. This dual excretory pathway is qualitatively different from that of other sulfonylureas, which are excreted primarily in the urine.
Metformin
Intravenous single-dose studies in normal subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) nor biliary excretion. Renal clearance (see Table 4) is approximately 3.5 times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
Specific Populations
Hepatic Impairment
No pharmacokinetic studies have been conducted in patients with hepatic insufficiency for either glyburide or metformin [see Warnings and Precautions (8.7)].
Renal Impairment
No information is available on the pharmacokinetics of glyburide in patients with renal insufficiency.
In patients with decreased renal function the plasma and blood half-life of metformin is prolonged and the renal clearance is decreased (Table 4); [see Dosage and Administration (2), Contraindications (4), and Warnings and Precautions (5.1)].
Geriatrics
There is no information on the pharmacokinetics of glyburide in elderly patients.
Limited data from controlled pharmacokinetic studies of metformin in healthy elderly subjects suggest that total plasma clearance is decreased, the half-life is prolonged, and Cmax is increased, when compared to healthy young subjects. From these data, it appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function (Table 4); [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
| Table 4: Select Mean (±SD) Metformin Pharmacokinetic Parameters Following
Single or Multiple Oral Doses of Metformin HCl | |||
| Subject Groups: Metformin HCl Dosea
(number of subjects) | Cmaxb
(µg/mL) | Tmaxc
(hrs) | Renal Clearance
(mL/min) |
| Healthy, nondiabetic adults: | |||
| 500 mg SDd(24) | 1.03 (±0.33) | 2.75 (±0.81) | 600 (±132) |
| 850 mg SD (74)e | 1.60 (±0.38) | 2.64 (±0.82) | 552 (±139) |
| 850 mg t.i.d. for 19 dosesf (9) | 2.01 (±0.42) | 1.79 (±0.94) | 642 (±173) |
| Adults with type 2 diabetes: | |||
| 850 mg SD (23) | 1.48 (±0.5) | 3.32 (±1.08) | 491 (±138) |
| 850 mg t.i.d. for 19 dosef (9) | 1.90 (±0.62) | 2.01 (±1.22) | 550 (±160) |
| Elderlyg, healthy nondiabetic adults: | |||
| 850 mg SD (12) | 2.45 (±0.70) | 2.71 (±1.05) | 412 (±98) |
| Renal-impaired adults: 850 mg SD | |||
| Mild (CLcrh 61-90 mL/min) (5) | 1.86 (±0.52) | 3.20 (±0.45) | 384 (±122) |
| Moderate (CLcr 31-60 mL/min) (4) | 4.12 (±1.83) | 3.75 (±0.50) | 108 (±57) |
| Severe (CLcr 10-30 mL/min) (6) | 3.93 (±0.92) | 4.01 (±1.10) | 130 (±90) |
| a All doses given fasting except the first 18 doses of the multiple-dose studies | |||
| b Peak plasma concentration | |||
| c Time to peak plasma concentration | |||
| d SD=single dose | |||
| e Combined results (average means) of 5 studies: mean age 32 years (range 23-59 years) | |||
| f Kinetic study done following dose 19, given fasting | |||
| g Elderly subjects, mean age 71 years (range 65-81 years) | |||
| h CLcr=creatinine clearance normalized to body surface area of 1.73 m2 |
Gender
There is no information on the effect of gender on the pharmacokinetics of glyburide.
Metformin pharmacokinetic parameters did not differ significantly in subjects with or without type 2 diabetes when analyzed according to gender (males=19, females=16).
Race
No information is available on race differences in the pharmacokinetics of glyburide.
No studies of metformin pharmacokinetic parameters according to race have been performed.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal studies have been conducted with the combined products in GLUCOVANCE. The following data are based on findings in studies performed with the individual products.
Glyburide
Studies in rats with glyburide alone at doses up to 300 mg/kg/day (approximately 145 times the maximum recommended human daily dose of 20 mg for the glyburide component of GLUCOVANCE based on body surface area comparisons) for 18 months revealed no carcinogenic effects. In a 2-year oncogenicity study of glyburide in mice, there was no evidence of treatment-related tumors.
There was no evidence of mutagenic potential of glyburide alone in the following in vitro tests: Salmonella microsome test (Ames test) and in the DNA damage/alkaline elution assay.
No evidence of impaired fertility was observed when doses up to 500 times the maximum recommended human dose of 20 mg of glyburide, based on body surface area comparisons, were administered to rats in reproduction studies.
Metformin
Long-term carcinogenicity studies have been performed in rats (dosing duration of 104 weeks) and mice (dosing duration of 91 weeks) at doses up to and including 900 mg/kg/day and 1500 mg/kg/day, respectively. These doses are both approximately 4 times the maximum recommendation human daily dose of 2000 mg on body surface area comparisons. No evidence of carcinogenicity with metformin was found in either male or female mice. Similarly, there was no tumorigenic potential observed with metformin in male rats. There was, however, an increased incidence of benign stromal uterine polyps in female rats treated with 900 mg/kg/day.
There was no evidence of a mutagenic potential of metformin in the following in vitro tests: Ames test (S. typhimurium), gene mutation test (mouse lymphoma cells), or chromosomal aberrations test (human lymphocytes). Results in the in vivo mouse micronucleus test were also negative.
Fertility of male or female rats was unaffected by metformin when administered at doses as high as 600 mg/kg/day, which is approximately 3 times the maximum recommended human daily dose of 2000 mg based on body surface area comparisons.
14. Clinical Studies
Patients with Inadequate Glycemic Control on Diet and Exercise Alone
In a 20-week, double-blind, placebo-controlled, multicenter U.S. clinical study, involving 806 drug-naive patients with type 2 diabetes, whose hyperglycemia was not adequately controlled with dietary management alone (baseline fasting plasma glucose [FPG] below 240 mg/dL, baseline hemoglobin A1c [HbA1c] between 7% and 11%), were randomized to receive initial therapy with placebo, 2.5 mg glyburide, 500 mg metformin HCl, GLUCOVANCE 1.25 mg/250 mg, or GLUCOVANCE 2.5 mg/500 mg. After 4 weeks, the dose was progressively increased to a maximum of 4 tablets daily as needed to reach a target FPG of 126 mg/dL. Study data at 20 weeks are summarized in Table 5.
| Table 5: Mean Change in Hemoglobin A1c and Fasting Plasma Glucose in Patients Receiving Placebo, Glyburide, Metformin HCl or GLUCOVANCE at 20 Weeks | |||||
| Placebo |
Glyburide 2.5 mg tablets |
Metformin HCl 500 mg tablets |
GLUCOVANCE 1.25 MG/250 mg tablets |
GLUCOVANCE 2.5 mg/500 mg tablets |
|
| Mean Final Dose | 0 mg | 5.3 mg | 1317 mg | 2.78 mg/557 mg | 4.1 mg/824 mg |
| Hemoglobin A1c | N=147 | N=142 | N=141 | N=149 | N=152 |
| Baseline Mean (%) | 8.14 | 8.14 | 8.23 | 8.22 | 8.20 |
| Mean Change from Baseline | -0.21 | -1.24 | -1.03 | -1.48 | -1.53 |
| Difference from Placebo | -1.02 | -0.82 | -1.26a | -1.31a | |
| Difference from Glyburide | -2.4b | -0.29a | |||
| Difference from Metformin | -0.44b | -0.49b | |||
| Fasting Plasma Glucose | N=159 | N=158 | N=156 | N=153 | N=154 |
| Baseline Mean FPG (mg/dL) | 177.2 | 178.9 | 175.1 | 178 | 176.6 |
| Mean Change from Baseline | 4.6 | -35.7 | -21.2 | -41.5 | -40.1 |
| Difference from Placebo | -40.3 | -25.8 | -46.1a | -44.7a | |
| Difference from Glyburide | -5.8c | -4.5c | |||
| Difference from Metformin | -20.3c | -18.9c | |||
| Final HbA1c Distribution (%) | N-147 | N=142 | N=141 | N=149 | N=152 |
| <7% | 19.7% | 59.9% | 50.4% | 66.4% | 71.7% |
| ≥7% and <8% | 37.4% | 26.1% | 29.8% | 25.5% | 19.1% |
| ≥8% | 42.9% | 14.1% | 19.9% | 8.1% | 9.2% |
| a p<0.001 | |||||
| b p<0.05 | |||||
| c p=NS | |||||
Mean baseline body weight was 87 kg, 87 kg, 89 kg, 89 kg and 87 kg in the placebo, glyburide 2.5mg, metformin 500mg, GLUCOVANCE 1.25mg/250mg and 2.5mg/500mg arms, respectively. Mean change in body weight from baseline to week 20 was -0.7 kg, +1.7 kg, -0.6 kg, +1.4 kg and +1.9 in the placebo, glyburide, metformin, GLUCOVANCE 1.25mg/250mg and 2.5mg/500mg arms, respectively.
Patients with Inadequate Glycemic Control on Sulfonylurea Alone
In a 16-week, double-blind, active-controlled U.S. clinical study, a total of 639 patients with type 2 diabetes not adequately controlled (mean baseline HbA1c 9.5%, mean baseline FPG 213 mg/dL) while being treated with at least one-half the maximum dose of a sulfonylurea (e.g., glyburide 10 mg, glipizide 20 mg) were randomized to receive glyburide (fixed dose, 20 mg), metformin HCl (500 mg), GLUCOVANCE 2.5 mg/500 mg, or GLUCOVANCE 5 mg/500 mg. The doses of metformin HCl and GLUCOVANCE were titrated to a maximum of 4 tablets daily as needed to achieve FPG <140 mg/dL. Study data at 16 weeks are summarized in Table 6.
| Table 6: Mean Change in Hemoglobin A1c and Fasting Plasma Glucose in Patients Receiving Glyburide, Metformin HCl or GLUCOVANCE at 16 Weeks | ||||
|
Glyburide 5 mg tablets |
Metformin HCl 500 mg tablets |
GLUCOVANCE 2.5 mg/500 mg tablets |
GLUCOVANCE 5 mg/500 mg tablets |
|
| Mean Final Dose | 20 mg | 1840 mg | 8.8 mg/1760 mg | 17 mg/1740 mg |
| Hemoglobin A1c | N=158 | N=142 | N=154 | N=159 |
| Baseline Mean (%) | 9.63 | 9.51 | 9.43 | 9.44 |
| Final Mean | 9.61 | 9.82 | 7.92 | 7.91 |
| Difference from Glyburide | -1.69a | -1.70a | ||
| Difference from Metformin | -1.90a | -1.91a | ||
| Fasting Plasma Glucose | N=163 | N=152 | N=160 | N=160 |
| Baseline Mean (mg/dL) | 218.4 | 213.4 | 212.2 | 210.2 |
| Final Mean | 221.0 | 233.8 | 169.6 | 161.1 |
| Difference from Glyburide | -51.3a | -59.9a | ||
| Difference from Metformin | -64.2a | -72.7a | ||
| Final HbA1c Distribution (%) | N=158 | N=142 | N=154 | N=159 |
| <7% | 2.5% | 2.8% | 24.7% | 22.6% |
| ≥7% and <8% | 9.5% | 11.3% | 33.1% | 37.1% |
| ≥8% | 88% | 85.9% | 42.2% | 40.3% |
| a p<0.001 | ||||
Weight gain due to glyburide was comparable in all three exposed groups.
16. How is Glucovance supplied
GLUCOVANCE tablets are available as follows:
| Glyburide | Metformin HCl | Bottle size | NDC | Tablet Appearance |
| 2.5 mg | 500 mg | Bottles of 100 | 0087-6073-11 | pale orange, capsule-shaped, bevel-edged, biconvex, film-coated tablet with "BMS" debossed on one side and "6073" debossed on the opposite side |
| 5 mg | 500 mg | Bottles of 100 | 0087-6074-11 | yellow, capsule-shaped, bevel-edged, biconvex, film-coated tablet with "BMS" debossed on one side and "6074" debossed on the opposite side. |
Store at temperatures up to 25 °C (77 °F). [See USP Controlled Room Temperature.]
Dispense in light-resistant containers.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Lactic Acidosis:
Explain the risks of lactic acidosis, its symptoms, and conditions that predispose to its development. Advise patients to discontinue GLUCOVANCE immediately and to promptly notify their healthcare provider practitioner if unexplained hyperventilation, myalgia, malaise, unusual somnolence, or other nonspecific symptoms occur. Counsel patients against excessive alcohol intake and inform patients about importance of regular testing of renal function while receiving GLUCOVANCE.
Instruct patients to inform their doctor that they are taking GLUCOVANCE prior to any surgical or radiological procedure, as temporary discontinuation may be required [see Warnings and Precautions (5.1)].
Hypoglycemia:
Inform patients that hypoglycemia may occur when taking GLUCOVANCE. Explain to patients the risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development [see Warnings and Precautions (5.2)].
Cardiovascular Mortality:
Inform patients that the administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. Inform patients of the potential risks and benefits of glyburide and of alternative modes of therapy [see Warnings and Precautions (5.3)].
Vitamin B12 Deficiency:
Inform patients about importance of regular hematological testing while receiving GLUCOVANCE [see Warnings and Precautions (5.5)].
Females of Reproductive Age:
Inform females that treatment with GLUCOVANCE may result in ovulation in some premenopausal anovulatory women which may lead to unintended pregnancy [see Use in Specific Populations (8.3)].
Distributed by:
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA
GLUCOVANCE® and GLUCOPHAGE® are registered trademarks of Merck Santé S.A.S., a subsidiary of Merck KGaA of Darmstadt, Germany. Licensed to Bristol-Myers Squibb Company.
[print code TBD]
Rev [December 2018]
PATIENT INFORMATION
GLUCOVANCE® [GLOO-KŌ-VANZ]
(glyburide and metformin hydrochloride) tablets, for oral use
What is the most important information I should know about GLUCOVANCE?
GLUCOVANCE can cause serious side effects, including:
Lactic Acidosis. Metformin hydrochloride, a medicine in GLUCOVANCE, can cause a rare, but serious, side effect called lactic acidosis (a build-up of lactic acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in a hospital.
Stop taking GLUCOVANCE and call your healthcare provider right away if you get any of the following symptoms of lactic acidosis:
|
|
|
|
|
|
|
|
You have a higher chance of getting lactic acidosis if you:
- have severe kidney problems. See "Do not take GLUCOVANCE if you:"
- have liver problems.
- drink a lot of alcohol (very often or short-term "binge" drinking).
- get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting, or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and do not drink enough fluids.
- have certain x-ray tests with injectable dyes or contrast agents.
- have surgery or other procedures for which you need to restrict the amount of food and liquid you eat and drink.
- have congestive heart failure.
- have a heart attack, severe infection, or stroke.
- are 65 years of age or older.
Tell your healthcare provider if you have any of the problems in the list above.
Tell your healthcare provider that you are taking GLUCOVANCE before you have surgery or x-ray tests.
Your healthcare provider may need to stop GLUCOVANCE for a while if you have surgery or certain x-ray tests.
GLUCOVANCE can have other serious side effects. See "What are the possible side effects of GLUCOVANCE?"
What is GLUCOVANCE?
- GLUCOVANCE is a prescription medicine that contains glyburide (sulfonylurea) and metformin hydrochloride. GLUCOVANCE is used with diet and exercise to help control high blood sugar (hyperglycemia) in adults with type 2 diabetes.
- It is not known if GLUCOVANCE is safe and effective in children under 18 years of age.
Do not take GLUCOVANCE if you:
- have severe kidney problems.
- are allergic to metformin hydrochloride, glyburide or any of the ingredients in GLUCOVANCE. See the end of this Patient Information leaflet for a complete list of ingredients in GLUCOVANCE.
- have a condition called metabolic acidosis, including diabetic ketoacidosis (high levels of certain acids called "ketones" in your blood or urine).
- take bosentan.
Before taking GLUCOVANCE tell your healthcare provider about all medical conditions, including if you:
- have a history or risk for diabetic ketoacidosis. See "Do not take GLUCOVANCE if you:"
- have kidney problems.
- have liver problems.
- have heart problems, including congestive heart failure.
- are 65 year of age or older.
- drink alcohol very often, or drink a lot of alcohol in short-term "binge" drinking.
- have or any members of your family have glucose-6-phosphate dehydrogenase (G6PD) deficiency.
- are taking insulin or another sulfonylurea medicine.
- are pregnant or plan to become pregnant. It is not known if GLUCOVANCE will harm your unborn baby. You should not take GLUCOVANCE during the last 2 weeks of your pregnancy. If you are pregnant, talk with your healthcare provider about the best way to control your blood sugar while you are pregnant.
- are a woman who has not gone through menopause (premenopausal) who does not have periods regularly or at all. GLUCOVANCE can cause the release of an egg from an ovary in a woman (ovulation). This can increase your chance of getting pregnant.
- are breastfeeding or plan to breastfeed. It is not known if glyburide one of the medicines in GLUCOVANCE passes into your breast milk. Metformin the other medicine in GLUCOVANCE can pass into your breastmilk. Talk with your healthcare provider about the best way to feed your baby while you take GLUCOVANCE.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
GLUCOVANCE may affect the way other medicines work, and other medicines may affect how GLUCOVANCE works.
How should I take GLUCOVANCE?
- Take GLUCOVANCE exactly as your healthcare provider tells you.
- GLUCOVANCE should be taken 2 times each day with meals to help decrease an upset stomach side effect and avoid hypoglycemia.
- If you are taking colesevelam and GLUCOVANCE, take your GLUCOVANCE at least 4 hours before taking your colesevelam.
- Your healthcare provider should do blood tests to check how well your kidneys are working before and during your treatment with GLUCOVANCE.
- Your healthcare provider will check your diabetes with regular blood tests, including your blood sugar levels and your hemoglobin A1c.
- Low blood sugar (hypoglycemia) can happen more often when GLUCOVANCE is taken with certain other diabetes medicines. Talk to your healthcare provider about how to prevent, recognize, and manage low blood sugar. See "What are the possible side effects of GLUCOVANCE?"
- Check your blood sugar as your healthcare provider tells you to.
- If you take too much GLUCOVANCE, call your healthcare provider or go to the nearest hospital emergency room
right away.
What should I avoid while taking GLUCOVANCE?
- Do not drink a lot of alcoholic drinks while taking GLUCOVANCE. This means you should not binge drink for short periods, and you should not drink a lot of alcohol on a regular basis. Alcohol can increase the chance of getting lactic acidosis.
- Do not drive, operate machinery, or do other dangerous activities until you know how GLUCOVANCE affects you.
What are the possible side effects of GLUCOVANCE?
GLUCOVANCE can cause serious side effects, including:
- See "What is the most important information I should know about GLUCOVANCE?"
- low blood sugar (hypoglycemia). Low blood sugar is a serious, but common side effect of GLUCOVANCE. If you take GLUCOVANCE with another medicine that can cause low blood sugar, such as insulin, you have a higher risk of getting low blood sugar. The dose of insulin or other diabetic medicines may need to be lowered while you take GLUCOVANCE. Signs and symptoms of low blood sugar may include:
| o headache | o hunger | o dizziness |
| o drowsiness | o fast heartbeat | o sweating |
| o weakness | o confusion | o blurred vision |
| o irritability | o shaking or feeling jittery | o anxiety |
| o slurred speech | o mood changes |
- increased risk of cardiovascular deaths. Taking oral hypoglycemic medicines like GLUCOVANCE to treat diabetes have increased the risk of death from heart disease or stroke compared to treating diabetes with diet alone or diet and insulin.
- hemolytic anemia. People with G6PD deficiency who take GLUCOVANCE may develop hemolytic anemia (fast breakdown of red blood cells).
- low vitamin B12 (vitamin B12 deficiency). Using GLUCOVANCE may cause a decrease in the amount of vitamin B12 in your blood, especially if you have had low vitamin B12 levels before. Your healthcare provider may do blood tests to check your vitamin B12 levels.
The most common side effects of GLUCOVANCE include:
| o diarrhea | o vomiting |
| o headache | o stomach pain |
| o nausea | o dizziness |
These are not all the possible side effects of GLUCOVANCE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store GLUCOVANCE?
- Store GLUCOVANCE at room temperature between 68°F to 77°F (20°C to 25°C).
Keep GLUCOVANCE and all medicines out of the reach of children.
General information about the safe and effective use GLUCOVANCE.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use GLUCOVANCE for a condition for which it was not prescribed. Do not give GLUCOVANCE to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about GLUCOVANCE that is written for health professionals.
What are the ingredients in GLUCOVANCE?
Active ingredients: glyburide and metformin hydrochloride.
Inactive ingredients: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and povidone.
GLUCOVANCE® is a registered trademark of Merck Santé S.A.S., a subsidiary of Merck KGaA of Darmstadt, Germany. Licensed to Bristol-Myers Squibb Company.
Other brands listed are the trademarks of their respective owners.
Distributed by:
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA
This Patient Package Insert has been approved by the U.S. Food and Drug Administration Revison: 12/2018
| GLUCOVANCE
glyburide and metformin hydrochloride tablet, film coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| GLUCOVANCE
glyburide and metformin hydrochloride tablet, film coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Bristol-Myers Squibb Company (102826703) |
More about Glucovance (glyburide / metformin)
- Check interactions
- Compare alternatives
- Reviews (2)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: antidiabetic combinations



