Folene: Package Insert / Prescribing Info
Package insert / product label
Generic name: folate
Dosage form: capsule
Drug class: Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Mar 24, 2025.
On This Page
Folene Description
Folene™ is an orally administered prescription-folate preparation specifically formulated for the dietary management of patients with nutritional needs requiring increased folate and magnesium levels. Specifically, Folene™ is formulated for patients in need of increased clinical dietary management providing necessary cofactors: Vitamin D, magnesium and calcium. Softgels must be administered under the supervision of a licensed medical practitioner to monitor/rule-out pernicious anemia (cobalamin) deficiency.
INGREDIENTS:
Each serving of (2) light blue Softgels contains the following ingredients:
L-methylfolate magnesium ..... 2 mg
Vitamin D3 ..... 3775 IU
NAC:Magnesium ..... 100 mg
Magnesium Citrate..... 500 mg
Calcium Citrate..... 200 mg
Excipients: Soybean Oil, Gelatin (Bovine), Glycerin, Lecithin, Purified Water, Beeswax, Citric Acid, Titanium Dioxide, Natural Creamy Orange Flavor, Ascorbic Acid, L-Glutathione, and FD&C Blue #1. CAUTION: Contains Bovine Gelatin and Soy
MECHANISM OF ACTION:
Folates are best known for reducing the incidence of fetal neural tube defects (NTDs).1,2,3 NTDs are congenital malformations produced by failure of the neural tube to form and close properly during embryonic development.3,4 During the first four weeks of pregnancy – when many women do not even realize that they have conceived, adequate maternal folate intake is reported to reduce the risk of NTDs. Folate is also essential in the synthesis and maintenance of nucleoprotein in erythropoiesis. It also promotes white blood cell (WBC) and platelet production in folate-deficiency anemia. Folate is associated with methylation and transformylation biochemistry. Folate is involved in transformylation and methylation metabolism as well as, indirectly, succinylation metabolism (through the “methyl trap” hypothesis). Folate plays a central role in the formation of nucleic acid precursors, such as thymidylic acid and purine nucleotides, which are essential for nucleic acid synthesis and cell division. IOM/NAS (1998) noted that the evidence for a protective effect from folate supplements is much stronger than that for food folate .3
Folene - Clinical Pharmacology
FOLATE is essential for the production of certain coenzymes in many metabolic systems such as purine and pyrimidine synthesis. About 70% of food folate and cellular folate is comprised of L-methylfolate. It is the primary form of folate in circulation, and is also the form transported across membranes – particularly across the blood brain barrier – into peripheral tissues. In the cell, L-methylfolate is used in the re-methylation of homocysteine to form methionine and tetrahydrofolate (THF). It has been reported that L-methylfolate supplementation increases the formation of endothelial nitric oxide synthase resulting in increase nitric oxide. 5 This causes vasodilation to the nerves, increasing blood supply to the nerves, and reducing vascular oxidative stress. 5 L-methylfolate supplementation is also reported to increase nitric oxide levels by increasing the amount of tetrahydrobiopterin (BH4). BH4 is required for nitric oxide synthesis.6 The combination of Vitamin D, magnesium, and calcium have been shown to provide a synergistic affect in elevating blood levels for such deficiencies. 10
VITAMIN D regulates calcium homeostasis by maintaining equilibrium (along with parathormone) between calcium resorption and excretion.7 Adequate calcium intake increases Vitamin D levels.7,8 Vitamin D is a fat-soluble vitamin. Unlike water-soluble vitamins, fat-soluble vitamins dissolve in fat and are stored in body tissues. Both Vitamin D and magnesium are required for proper calcium deposition and bone development. 9
MAGNESIUM plays an important role in biochemical reactions all over the body. It is involved in many cell transport activities, in addition to helping cells make energy aerobically or anaerobically. Bones are a major reservoir for magnesium, and magnesium is the counter-ion for calcium and potassium in muscle cells, including the heart. 10 Magnesium also is designed to offset calcium. Magnesium contributes to the maintenance of normal bone and teeth. 11 The combination of high doses of Vitamin D and magnesium supplementation improves magnesium absorption and utility. 8
Indications and Usage for Folene
Folene™ is a prescription-folate preparation indicated for the nutritional supplementation for individuals who have suboptimal folate, magnesium, and/or Vitamin D levels in the plasma and require a maintenance level. Folene should be administered only under that direction and supervision of a licensed medical practitioner.
Warnings
Caution is recommended in patients with a family history of bipolar illness. Mood elevation is possible in this population. Caution is also recommended in patients taking anticonvulsant medications as folate may interfere with anticonvulsant medication, and may lower seizure threshold. Furthermore, it has been reported that anticonvulsant medications interfere with folate metabolism, but the exact action is unclear; therefore caution is recommended with patients in this therapeutic group. Caution is recommended in patients with kidney disease. Patients undergoing cancer treatment should consult their licensed medical practitioner for advice.
Precautions
Folate alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folate in doses above 1.0 mg daily may obscure pernicious anemia in that hematologic remission may occur while neurological manifestations progress.
Contraindications
ADVERSE REACTIONS
Allergic reactions have been reported following the use of oral and parenteral folate. 14 Mild transient diarrhea, polycythemia vera, itching, transitory exanthema, and the feeling of swelling of the entire body have been associated with methylcobalamin. 15 Allergic reactions, acne, skin reactions, photosensitivity, nausea, vomiting, abdominal pain, loss of appetite, paresthesia, somnolence, nausea, and headaches have been associated with pyridoxal-5’-phosphate. 16 Call your licensed medical practitioner about side effects.
DRUG INTERACTIONS
This product is contraindicated in patients with a known hypersensitivity to any of the articles contained in this product. This product is contraindicated for individuals with conditions for which any of the ingredients are contraindicated. Talk to your licensed medical practitioner, healthcare practitioner, personal physician, and/or pharmacist before taking or using any prescription, over-the-counter medicines, or herbal/health supplements alongside this product.
Drugs which may interact with folate include:
- Antiepileptic drugs (AED): The AED class including, but not limited to, phenytoin, carbamazepine, primidone, valproic acid, fosphenytoin, valproate, phenobarbital and lamotrigine have been shown to impair folate absorption and increase the metabolism of circulating folate.
- Additionally, concurrent use of folic acid has been associated with enhanced phenytoin metabolism, lowering the level of the AED in the blood and allowing breakthrough seizures to occur. Caution should be used when prescribing this product among patients who are receiving treatment with phenytoin and other anticonvulsants.
- Cholestyramine, Colestipol, Cycloserine: Reduces folic acid absorption and reduces serum folate levels.
- Fluoxetine: Fluoxetine exerts a noncompetitive inhibition of the 5-methyltetrahydrofolate active transport in the intestine.
- Isotretinoin: Reduced folate levels have occurred in some patients taking isotretinoin.
- L-dopa, triamterene, colchicine, and trimethoprim may decrease plasma folate levels.
- Nonsteroidal Anti-inflammatory Drugs (NSAIDs): NSAIDs have been shown to inhibit some folate dependent enzymes in laboratory experiments.
- NSAIDs include ibuprofen, naproxen, indomethacin and sulindac.
- Oral Contraceptives: Serum folate levels may be depressed by oral contraceptive therapy.
- Methylprednisolone: Reduced serum folate levels have been noted after treatment with methylprednisolone.
- Pancreatic Enzymes: Reduced serum folate levels have occurred in some patients taking pancreatic extracts, such as pancreatin and pancrelipase.
- Pentamidine: Reduced folate levels have been seen with prolonged intravenous pentamidine.
- Pyrimethamine: High levels of folic acid may result in decreased serum levels of pyrimethamine.
- Smoking and Alcohol: Reduced serum folate levels have been noted.
- Sulfasalazine: Inhibits the absorption and metabolism of folic acid.
- Metformin treatment in patients with type 2 diabetes decreases serum folate.
- Warfarin can produce significant impairment in folate status after a 6-month therapy.
- Caution should be exercised with the concomitant use of folinic acid and tri-methoprim sulfamethoxazole for the acute treatment of Pneumocystis carinii pneumonia in patients with HIV infection as it is associated with increased rates of treatment failure and mortality in a placebo controlled study.
Drugs which may interact with Vitamin D (cholecalciferol):
- Calcipotriene (Dovonex) , Digoxin (Lanoxin), Diltiazem (Cardizem, Dilacor, Tiazac), Verapamil (Calan, Covera, Isoptin, Verelan), Water pills (Thiazide diuretics)
Related/similar drugs
Folene Dosage and Administration
ORAL ADMINISTRATION The usual adult dose may be taken as two (2) softgels once daily, or as directed by a licensed medical practitioner
How is Folene supplied
Folene™ softgels are light blue softgels with the imprint “F200”, and are supplied in bottles of 60 Softgels and are dispensed by prescription. Bottle of 60† NDC 58809-200-60 (60 ct. bottle / 60 Softgel).This product may, under certain circumstances, be dispensed through a certified mail-order program as long as there is record of prescription AND confirmation that the patient is under the supervision of a licensed medical practitioner
Storage and Handling
Store at Controlled Room Temperature 15°-30°C (59°-86°F). [See USP]. Protect from light and moisture. Dispense in a tight, light-resistant container. To report adverse side effects or to obtain product information, contact GM Pharmaceuticals, Inc. at 1-888-535-0305. Inform your medical practitioner about side effects. You may also report side effects to FDA at 1-800-FDA-1088.
These statements have not been reviewed or approved by the FDA. This product is not an Orange (OB) rated product.
KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN. RX ONLY.
FEDERAL LAW PROHIBITS DISPENSING WITHOUT A PRESCRIPTION.
MANUFACTURED FOR: GM Pharmaceuticals, Inc. Arlington, Texas 76015
PATENTS: U.S. Pat. No. 9,062,086 and other Patents Pending. TRADEMARKS: Folene TM is a trademark of GM
Pharmaceuticals, Inc.
FOLATE REGULATION
The Federal Register Notices from 1971 to 1973 establish that increased folate is a proper supplement in megaloblastic anemias of tropical and nontropical sprue, nutritional origin, pregnancy, infancy, and childhood.17,18 Folate metabolism may be affected by malabsorption issues that differ widely among population groups. The March 5, 1996 Federal Register Notice (61 FR 8760) states “The agency concluded that the scientific literature did not support the superiority of any one source of folate over others, and that the data were insufficient to provide a basis for stating that a specific amount of folate is more effective than another amount" [emphasis added].19 The Federal Register Notice of August 2, 1973 (38 FR 20750) states that “dietary supplement preparations are available without a prescription (21 CFR 121.1134). Levels higher than dietary supplement amounts are available only with a prescription. Oral preparations supplying more than 0.8 mg of folate per dosage unit would be restricted to prescription dispensing and that a dietary supplement furnishing 0.8 mg could be prescribed when a maintenance level of 0.8 mg per day was indicated18” AND ALSO IN THAT FEDERAL REGISTER THAT: • “It was concluded that there is no evidence that doses of folic acid greater than 1mg. daily have greater efficacy than do those of 1mg.” • “Pending review of the status of folic acid by the OTC vitamin-mineral drug panel pursuant to procedures established in S130.301, the FDA will continue on an interim basis its previous policy of regarding any preparation containing folic acid in excess of permitted food additive level as prescription drug.” The Federal Register Notice of August 2, 1973 (38 FR 20750) also states that patients should be kept under supervision of a licensed practitioner and adjustment of the maintenance level made if relapse appears imminent. In the presence of alcoholism, hemolytic anemia, anticonvulsant therapy, or chronic infection, the maintenance level
may need to be increased [emphasis added].17” In the Letter Regarding Dietary Supplement Health Claim for Folic Acid, Vitamin B6, and Vitamin B12 and Vascular Disease (Docket No. 99P-3029) dated November 28, 2000, FDA wrote “... high intakes of folate may partially and temporarily correct pernicious anemia while the neurological damage of vitamin B12 deficiency progresses. IOM/NAS (1998) set the UL for all adults of 1 mg per day because of devastating and irreversible neurological consequences of vitamin B12 deficiency, the data suggesting that pernicious anemia may develop at a younger age in some racial or ethnic groups, and the uncertainty about the extent of the occurrence of vitamin B12 deficiency in younger age groups (IOM/NAS, 1998) [emphasis added].20”
REFERENCES
1. Hendler SS, Rorvik D, eds. PDR for Nutritional Supplements. Montvale, NJ: Thomson Healthcare; 2001.
2. Hendler SS, Rorvik D. PDR for Nutritional Supplements. 2nd ed. Montvale, NJ. Physicians’ Desk Reference Inc; 2008.
3. Bendich A, Deckelbaum R. Preventive Nutrition: The Comprehensive Guide for Health Professionals. 2009. Dietary Supplement Facts Regulations at
http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ DietarySupplements/ucm2006823.htm.
4. Letter Regarding Dietary Supplement Health Claim for Folic Acid With Respect to Neural Tube Defects. October 10, 2000.
5. Veves A. et al. Diabetes 1998. 47:457-463.
6. Verhaar, MC. Atherosclerosis, Thrombosis, Vascular Biology, 2001.
7. Heaney R. Vitamin D and calcium interactions: functional outcomes. Am. J Clin. Nutr. 2008;88 p541-544.
8. Jeruszka-Bielak M. et al. Relationship Between Nutritional Habits and Hair Calcium Levels in Young Women. Bio Trace Elem Res. Mar. 2011,
1144:63-76.
9. Genuis S. et al. Combination of Micronutrients for Bone (COMB) Study: Bone Density after Macronutrient Intervention. Jrl Env. and Pub. Health.
2012 p1-10.
10. Rosanoff A. et al. Essential Nutrient Interactions: Does low or Suboptimal Magnesium Status Interact with Vitamin D and/or Calcium Status? Adv. Nutr.
2016;7:25-43.
11. Opinion on the substance of health claims related to magnesium on maintenance of bone. EFSA Panel on Dietetic Products, Nutrition, and Allergies
(NDA). EFSA J 2009b; 7: 1216.
12. Bitarafan S. et al. Dietary intake of nutrients and its correlation with fatigue in multiple sclerosis patients. Iran Neuro. 2014;13(1):28-32.
13. Oliveira de Cunha A. et al. Inadequacies in the habitual nutrient intakes of patients with metabolic syndrome: a cross-sectional study. Diabetol Metab
Syndr. (2016) 8:32.
14. Natural Standard Research Collaboration (NIH). Folate (folic acid) Monograph 2009.
15. Natural Standard Research Collaboration (NIH). Vitamin B12 Monograph 2009.
16. Alternative Medicine Review Vitamin B6 Monograph Volume 6, Number 1, 2001.
17. Federal Register Notice of April 9, 1971 (36 FR 6843).
18. Federal Register Notice of August 2, 1973 (38 FR 20750).
19. Federal Register Notice of March 5, 1996 (61 FR 8759-60).
20. Letter Regarding Dietary Supplement Health Claim for Folic Acid, Vitamin B6, and Vitamin B12 and Vascular Disease. November 28, 2000
R081816
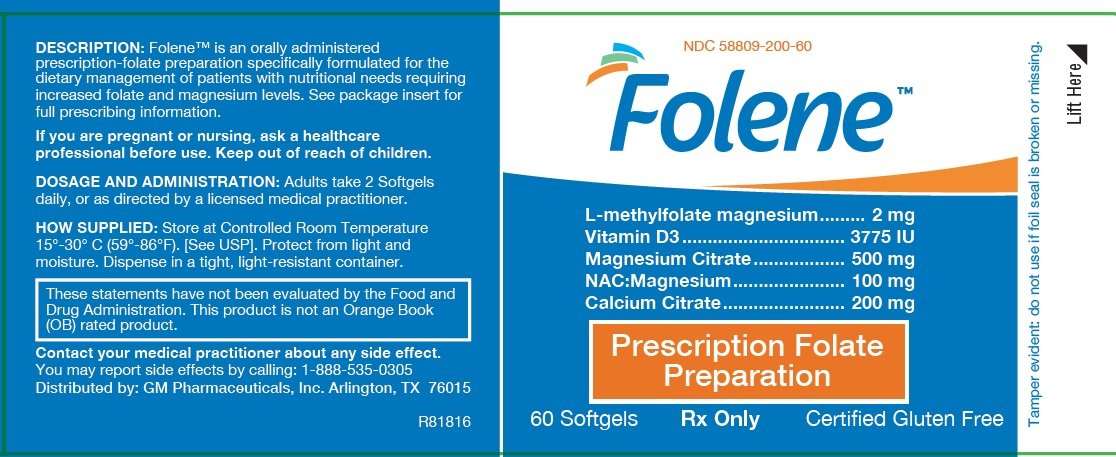
Principal Display Panel
Folene TM
L-methylfolate magnesium..... 2 mg
Vitamin D3 ..... 3775 IU
Magnesium Citrate..... 500 mg
NAC:Magnesium..... 100 mg
Calcium Citrate..... 200 mg
| Prescription Folate Preparation |
60 Softgels
Rx Only
Certified Gluten Free
|
These statements have not been reviewed or approved by the FDA. This product is not an Orange Book (OB) rated product. |
KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN. RX ONLY. FEDERAL LAW PROHIBITS DISPENSING WITHOUT A PRESCRIPTION.
MANUFACTURED FOR: GM Pharmaceuticals, Inc. Arlington, Texas 76015
PATENTS: U.S. Pat. No. 9,062,086 and other Patents Pending. TRADEMARKS: Folene™ is a trademark of GM Pharmaceuticals, Inc. NDC 58809-200-60

| FOLENE
prescription folate preparation capsule |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - GM Pharmaceuticals, Inc. (793000860) |
More about multivitamin with minerals
- Check interactions
- Compare alternatives
- Reviews (201)
- Drug images
- Side effects
- Dosage information
- Drug class: vitamin and mineral combinations
- En español
Patient resources
Professional resources
Other brands
Dolomite, Centratex, Bacmin, Dialyvite Supreme D, ... +8 more

