Strovite One: Package Insert / Prescribing Info
Package insert / product label
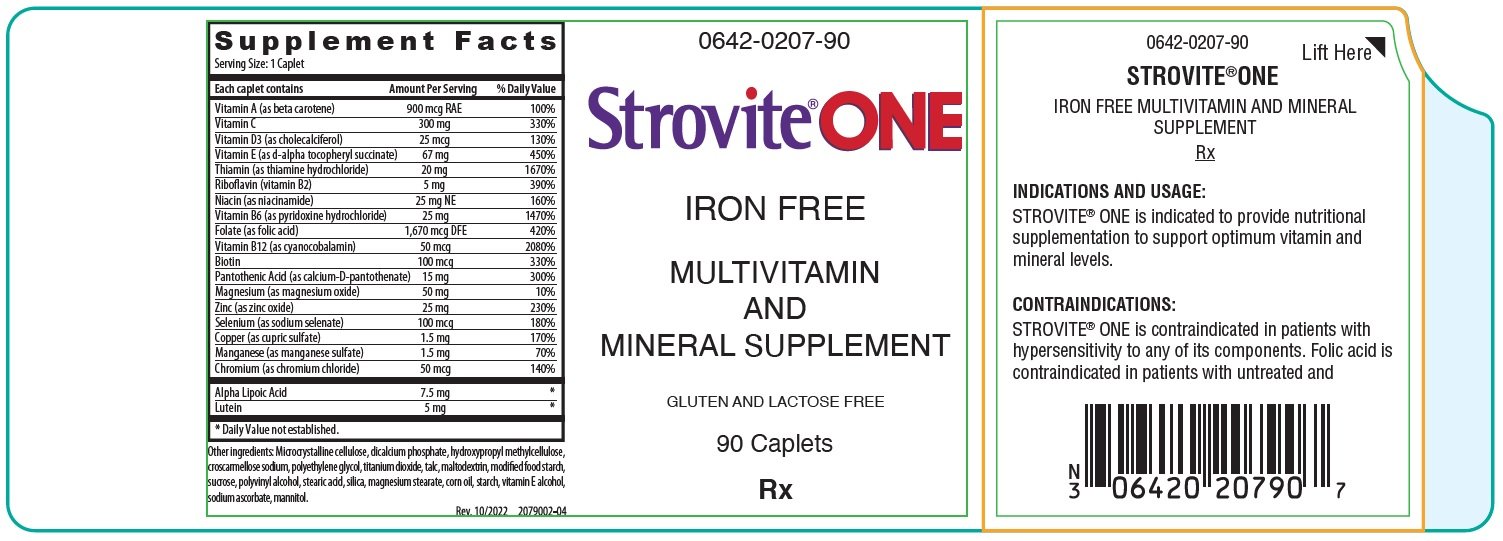
Generic name: iron free multivitamin and mineral supplement
Dosage form: tablet
Drug class: Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
On This Page
Strovite One Description
| Each caplet contains: | ||
| Vitamin A (as beta carotene) | 900 mcg RAE | |
| Vitamin C | 300 mg | |
| Vitamin D (as cholecalciferol) | 25 mcg | |
| Vitamin E (as d-alpha tocopheryl succinate) | 67 mg | |
| Thiamin (as thiamine hydrochloride) | 20 mg | |
| Riboflavin (vitamin B2) | 5 mg | |
| Niacin (as niacinamide) | 25 mg NE | |
| Vitamin B6 (as pyridoxine hydrochloride) | 25 mg | |
| Folate (as folic acid) | 1,670 mcg DFE | |
| Vitamin B12 (as cyanocobalamin) | 50 mcg | |
| Biotin | 100 mcg | |
| Pantothenic Acid (as calcium-D-pantothenate) | 15 mg | |
| Magnesium (as magnesium oxide) | 50 mg | |
| Zinc (as zinc oxide) | 25 mg | |
| Selenium (as sodium selenate) | 100 mcg | |
| Copper (as cupric sulfate) | 1.5 mg | |
| Manganese (as manganese sulfate) | 1.5 mg | |
| Chromium (as chromium chloride) | 50 mcg | |
| Alpha Lipoic Acid | 7.5 mg | |
| Lutein | 5 mg | |
Other ingredients: Microcrystalline cellulose, dicalcium phosphate, hydroxypropyl methylcellulose, croscarmellose sodium, polyethylene glycol, titanium dioxide, talc, maltodextrin, modified food starch, sucrose, polyvinyl alcohol, stearic acid, silica, magnesium stearate, corn oil, starch, vitamin E alcohol, sodium ascorbate, mannitol.
Indications and Usage for Strovite One
STROVITE ® ONE is indicated to provide nutritional supplementation to support optimum vitamin and mineral levels.
Contraindications
STROVITE ® ONE is contraindicated in patients with hypersensitivity to any of its components. Folic Acid is contraindicated in patients with untreated and uncomplicated pernicious anemia, and in those with anaphylactic sensitivity to folic acid.
Cyanocobalamin is contraindicated in patients with sensitivity to cobalt or to cyanocobalamin (Vitamin B12).
Warnings and Precautions
Vitamin D supplementation should be used with caution in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones. High doses of vitamin D can lead to elevated levels of calcium that reside in the blood and soft tissues. Bone pain, high blood pressure, formation of kidney stones, renal failure, and increased risk of heart disease can occur.
Folic acid, especially in doses above 0.1 mg daily, may obscure pernicious anemia, in that hematologic remission may occur while neurological manifestations remain progressive.
The use of folic acid doses above 1 mg daily may precipitate or exacerbate the neurological damage of vitamin B12 deficiency.
Avoid overdosage. Keep out of the reach of children.
Drug Interactions
High doses of folic acid may result in decreased serum levels of anticonvulsant drugs.
Vitamin D supplementation should not be given with large amounts of calcium in those with hypercalcemia or conditions that may lead to hyper-calcemia such as hyperparathyroidism and those who form calcium-containing kidney stones.
Zinc can inhibit the absorption of certain antibiotics; take at least 2 hours apart to minimize interactions. Consult appropriate references for additional specific vitamin-drug interactions.
Adverse Reactions/Side Effects
Adverse reactions have been reported with specific vitamins and minerals, but generally at levels substantially higher than those in STROVITE ® ONE.
Related/similar drugs
Strovite One Dosage and Administration
One caplet daily or as directed by a physician.
How is Strovite One supplied
STROVITE ® ONE is a white, oblong caplet, debossed EV0207; available in bottles of 90 caplets (0642-0207-90) and as professional samples (0642-0207-03).
| STROVITE ONE
CAPLETS
vitamin a, calcium pantothenate, ascorbic acid, cholecalciferol, .alpha.-tocopherol succinate, d-, thiamine, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, biotin, cyanocobalamin, selenium, magnesium oxide, zinc oxide, cupric sulfate, manganese, chromium, .alpha.-lipoic acid, and lutein tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Exeltis USA, Inc. (071170534) |
More about Strovite One (multivitamin with minerals)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- Drug class: vitamin and mineral combinations
Professional resources
Other brands
Dolomite, Centratex, Bacmin, Dialyvite Supreme D, ... +7 more