Combogesic IV: Package Insert / Prescribing Info
Package insert / product label
Generic name: acetaminophen and ibuprofen
Dosage form: injection
Drug class: Analgesic combinations
J Code (medical billing code): J0138 (IJ, acetaminophen 10 mg and ibuprofen 3 mg)
Medically reviewed by Drugs.com. Last updated on May 16, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
COMBOGESIC IV (acetaminophen and ibuprofen) injection for intravenous use
Initial U.S. Approval: 2023
WARNING: HEPATOTOXICITY, CARDIOVASCULAR RISK, and GASTROINTESTINAL RISK
See full prescribing information for complete boxed warning.
• Take care when prescribing, preparing, and administering COMBOGESIC IV to avoid dosing errors which could result in accidental overdose and death. (5.1)
• COMBOGESIC IV contains acetaminophen, which has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with doses of acetaminophen that exceed 4000 mg per day, and often involve more than one acetaminophen-containing product. (5.2)
• Nonsteroidal anti-inflammatory drugs (NSAIDS), like the ibuprofen in COMBOGESIC IV, may cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use. (5.3).
• COMBOGESIC IV is contraindicated in the setting of coronary artery bypass graft (CABG) surgery. (4, 5.3)
• NSAIDS, like the ibuprofen in COMBOGESIC IV, cause an increased risk of serious gastrointestinal (GI) adverse events, including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events. (5.4)
Indications and Usage for Combogesic IV
COMBOGESIC IV is indicated in adults where an intravenous route of administration is considered clinically
necessary for:
• the relief of mild to moderate pain
• the management of moderate to severe pain as an adjunct to opioid analgesics (1)
Limitations of Use
COMBOGESIC IV is indicated for short-term use of five days or less. (1)
Combogesic IV Dosage and Administration
• Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals. (2.1).
• Do not exceed the maximum total daily dose of COMBOGESIC IV (4,000 mg acetaminophen and 1,200 mg ibuprofen) in 24 hours. (2.1)
• Do not exceed a total daily dose of 4,000 mg (4 g) of acetaminophen from all sources. (2.1)
• Do not administer with other acetaminophen-containing products. (2.1)
• For adult patients weighing greater than or equal to 50 kg (actual body weight): The recommended dosage is 1,000 mg of acetaminophen and 300 mg of ibuprofen administered as a 15-minute infusion, every 6 hours, as necessary (2.2).
• For adult patients weighing less than 50 kg (actual body weight): The recommended dosage is 15 mg/kg acetaminophen and 4.5 mg/kg ibuprofen administered as a 15-minute infusion, every 6 hours, as necessary. (2.2)
Dosage Forms and Strengths
Injection: 1,000 mg/100 mL (10 mg/mL) of acetaminophen and 300 mg/100 mL (3 mg/mL) of ibuprofen in single-dose vial. (3)
Contraindications
COMBOGESIC IV is contraindicated in:
• patients who have previously demonstrated hypersensitivity to acetaminophen, ibuprofen, other NSAIDs or to any of the excipients in the IV formulation (4)
• patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients (4, 5.9, 5.11)
• the setting of coronary artery bypass graft (CABG) surgery (4, 5.3)
• patients with severe hepatic impairment or severe active liver disease (4)
Warnings and Precautions
• Hypertension: Patients taking some antihypertensive medications may have impaired response to these therapies when taking NSAIDs. Monitor blood pressure. (5.5)
• Heart Failure and Edema: Avoid use of COMBOGESIC IV in patients with severe heart failure unless benefits are expected to outweigh risk of worsening heart failure. (5.6)
• Renal Toxicity: Long-term administration of NSAIDs, including the ibuprofen component of COMBOGESIC IV, has resulted in renal papillary necrosis and other renal injury. (5.7)
• Anaphylactic Reactions: Discontinue use immediately if symptoms occur. (5.8)
• Exacerbation of Asthma Related to Aspirin Sensitivity: COMBOGESIC IV is contraindicated in patients with aspirin-sensitive asthma. Monitor patients with preexisting asthma (without aspirin sensitivity). (5.9)
• Serious Skin Reactions: Discontinue COMBOGESIC IV at first appearance of skin rash or other signs of hypersensitivity. (5.10)
• Drug Rash with Eosinophilia and Systemic Symptoms (DRESS): Discontinue and evaluate clinically. (5.11)
• Fetal Toxicity: Limit use of NSAID-containing products, including COMBOGESIC IV, between about 20 to 30 weeks in pregnancy due to the risk of oligohydramnios/fetal renal dysfunction. Avoid use of NSAID-containing products, including COMBOGESIC IV, in women at about 30 weeks gestation and later in pregnancy due to the risks of oligohydramnios/fetal renal dysfunction and premature closure of the fetal ductus arteriosus. (5.12)
• Hematologic Toxicity: Monitor hemoglobin or hematocrit in patients with any signs or symptoms of anemia (5.13).
Adverse Reactions/Side Effects
The most common adverse reactions (greater than or equal to 3%) are infusion site pain, nausea, constipation, dizziness, infusion site extravasation, vomiting, headache, somnolence. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
A number of known or potential interactions between COMBOGESIC IV and other drugs/drug classes exist. Please refer to the Drug Interactions section (7) for further information.
Use In Specific Populations
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2024
Full Prescribing Information
BOXED WARNING
WARNING: HEPATOTOXICITY, CARDIOVASCULAR RISK, and GASTROINTESTINAL RISK
RISK OF MEDICATION ERRORS: Take care when prescribing, preparing, and administering COMBOGESIC IV to avoid dosing errors which could result in accidental overdose and death (5.1).
HEPATOTOXICITY: COMBOGESIC IV contains acetaminophen. Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with doses of acetaminophen that exceed 4,000 mg per day, and often involve more than one acetaminophen-containing product [see Warnings and Precautions (5.2)].
CARDIOVASCULAR RISK: COMBOGESIC IV contains ibuprofen, a nonsteroidal anti-inflammatory drug (NSAID). NSAIDs cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may
increase with duration of use [see Warnings and Precautions (5.3)].
COMBOGESIC IV is contraindicated for treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery [see Contraindications (4) and Warnings and Precautions (5.3)].
GASTROINTESTINAL RISK: NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events [see Warnings and Precautions (5.4)].
1. Indications and Usage for Combogesic IV
COMBOGESIC IV is indicated in adults where an intravenous route of administration is considered clinically necessary for:
• the relief of mild to moderate pain
• the management of moderate to severe pain as an adjunct to opioid analgesics
Limitations of Use
COMBOGESIC IV is indicated for short-term use of five days or less.
2. Combogesic IV Dosage and Administration
2.1 Important Dosage and Administration Instructions
• Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions (5.4)].
• Do not exceed the maximum total daily dose of COMBOGESIC IV (4,000 mg acetaminophen and 1,200 mg ibuprofen) in 24 hours.
• Do not exceed a total daily dose of 4,000 mg (4 g) acetaminophen from all sources.
• Do not co-administer COMBOGESIC IV with other acetaminophen or ibuprofen containing products [see Warnings and Precautions (5.2)].
• Visually inspect for particulate matter and discoloration prior to administration. If visibly opaque particles, discoloration, or other foreign particulates are observed, do not use.
• Use COMBOGESIC IV in one patient on one occasion only. It contains no antimicrobial preservative. Discard any unused solution.
• Do not mix with diluents or with other medicines.
2.2 Recommended Dosage
For adult patients weighing greater than or equal to 50 kg (actual body weight): The recommended dosage of COMBOGESIC IV is one vial (100 mL; acetaminophen 1,000 mg/ibuprofen 300 mg) administered as a 15-minute infusion every 6 hours, as necessary.
For adult patients weighing less than 50 kg (actual body weight): The recommended dosage is 15 mg/kg acetaminophen and 4.5 mg/kg ibuprofen, administered as a 15-minute infusion every 6 hours, as necessary. This equates to a maximum single dose of 750 mg acetaminophen and 225 mg ibuprofen (discard remaining medicine in vial), and a total daily dose of 3,000 mg (3 g) acetaminophen and 900 mg ibuprofen.
2.3 Instructions for Intravenous Administration
• Administer as a 15-minute intravenous infusion.
• Do not mix other medications with the COMBOGESIC IV vial or infusion device.
• As for all solutions for infusion presented in glass vials, monitor closely, particularly at the end of infusion, regardless of administration route, in order to avoid air embolism. This applies particularly for central route infusion.
• To decrease the likelihood of bung fragmentation or the bung being forced into the vial, use a syringe or giving set with a diameter equal to or below 0.8 mm for solution sampling and ensure that the bung is pierced at the location specifically designed for needle introduction (where the thickness of the bung is the lowest).
• The entire 100 mL container of COMBOGESIC IV is not intended for use in patients weighing less than 50 kg. For doses less than 1,000 mg acetaminophen and 300 mg ibuprofen, the appropriate dose must be withdrawn from the container and placed into a separate container prior to administration. Using aseptic technique, withdraw the appropriate dose (weight-based) from an intact sealed COMBOGESIC IV container and place the measured dose in a separate empty, sterile container (e.g., glass bottle, plastic intravenous container, or syringe) for intravenous infusion to avoid the inadvertent delivery and administration of the total volume of the commercially available container. COMBOGESIC IV is supplied in a single-dose container and the unused portion must be discarded.
3. Dosage Forms and Strengths
Injection: 1,000 mg/100 mL (10 mg/mL) of acetaminophen and 300 mg/100 mL (3 mg/mL) of ibuprofen in a clear, colorless solution in single-dose vial.
4. Contraindications
COMBOGESIC IV is contraindicated in:
• patients with a known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to acetaminophen, ibuprofen, other NSAIDs or to any other components of this product [see Warnings and Precautions (5.8, 5.10, 5.11)]
• patients with a history of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients [see Warnings and Precautions (5.8, 5.9)]
• in the setting of coronary artery bypass graft (CABG) surgery [see Warnings and Precautions (5.3)]
• patients with severe hepatic impairment or severe active liver disease [see Warnings and Precautions (5.2)]
5. Warnings and Precautions
5.1 Risk of Medication Errors
Take care when prescribing, preparing, and administering COMBOGESIC IV in order to avoid dosing errors which could result in accidental overdose and death. In particular, be careful to ensure that:
• the dose in milligrams (mg) and milliliters (mL) is not confused;
• the dosing is based on weight for patients under 50 kg;
• infusion pumps are properly programmed; and
• the total daily dose of acetaminophen from all sources does not exceed maximum daily limits [see Dosage and Administration (2)].
5.2 Hepatotoxicity
Acetaminophen
COMBOGESIC IV contains acetaminophen. Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4,000 mg per day, and often involve more than one acetaminophen-containing product.
The risk of acute liver failure is higher in individuals with underlying liver disease and in individuals who ingest alcohol while taking acetaminophen.
Ibuprofen
COMBOGESIC IV contains ibuprofen, a NSAID. Elevations of ALT or AST (three or more times the upper limit of normal [ULN]) have been reported in approximately 1% of NSAID-treated patients in clinical trials. In addition, rare, sometimes fatal, cases of severe hepatic injury, including fulminant hepatitis, liver necrosis, and hepatic failure have been reported.
Elevations of ALT or AST (less than three times ULN) may occur in up to 15% of patients treated with NSAIDs, including ibuprofen.
Clinical Recommendations
COMBOGESIC IV is contraindicated in patients with severe hepatic impairment or severe active liver disease. COMBOGESIC IV has not been studied in patients with impaired hepatic function. Use in these patients is not recommended [see Use in Specific Populations (8.6)].
If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), discontinue COMBOGESIC IV immediately, and perform a clinical evaluation of the patient.
5.3 Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, including myocardial infarction (MI) and stroke, which can be fatal. Based on available data, it is unclear that the risk for CV thrombotic events is similar for all NSAIDs. The relative increase in serious CV thrombotic events over baseline conferred by NSAID use appears to be similar in those with and without known CV disease or risk factors for CV disease. However, patients with known CV disease or risk factors had a higher absolute incidence of excess serious CV thrombotic events, due to their increased baseline rate. Some observational studies found that this increased risk of serious CV thrombotic events began as early as the first weeks of treatment. The increase in CV thrombotic risk has been observed most consistently at higher doses.
To minimize the potential risk for an adverse CV event in NSAID-treated patients, the lowest effective dose for the shortest duration possible should be used. Physicians and patients should remain alert for the development of such events, throughout the entire treatment course, even in the absence of previous CV symptoms. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID, such as ibuprofen, increases the risk of serious gastrointestinal (GI) events [see Warnings and Precautions (5.4)].
Status Post Coronary Artery Bypass Graft (CABG) Surgery
Two large, controlled clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10-14 days following CABG surgery found an increased incidence of myocardial infarction and stroke. NSAIDs are contraindicated in the setting of CABG [see Contraindications (4)].
Post-MI Patients
Observational studies conducted in the Danish National Registry have demonstrated that patients treated with NSAIDs in the post-MI period were at increased risk of reinfarction, CV-related death, and all-cause mortality beginning in the first week of treatment. In this same cohort, the incidence of death in the first year post-MI was 20 per 100 person years in NSAID-treated patients compared to 12 per 100 person years in non-NSAID exposed patients. Although the absolute rate of death declined somewhat after the first year post-MI, the increased relative risk of death in NSAID users persisted over at least the next four years of follow-up.
Avoid the use of COMBOGESIC IV in patients with a recent MI unless the benefits are expected to outweigh the risk of recurrent CV thrombotic events. If COMBOGESIC IV is used in patients with a recent MI, monitor patients for signs of cardiac ischemia.
5.4 Gastrointestinal Bleeding, Ulceration, and Perforation
NSAIDs, including ibuprofen, cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the esophagus, stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients who develop a serious upper GI adverse event on NSAID therapy is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occurred in approximately 1% of patients treated for 3-6 months and in about 2-4% of patients treated for one year. However, even short-term therapy is not without risk.
Risk Factors for GI Bleeding, Ulceration and Perforation
Patients with a prior history of peptic ulcer disease and/or GI bleeding who used NSAIDs had a greater than 10-fold increased risk for developing a GI bleed compared to patients without these risk factors. Other factors that increase the risk of GI bleeding in patients treated with NSAIDs include longer duration of NSAID therapy; concomitant use of oral corticosteroids, aspirin, anticoagulants, or selective serotonin reuptake inhibitors (SSRIs); smoking; use of alcohol; older age; and poor general health status. Most post-marketing reports of fatal GI events occurred in elderly or debilitated patients. Additionally, patients with advanced liver disease and/or coagulopathy are at increased risk for getting an ulcer or bleeding.
Strategies to Minimize the GI Risks in NSAID-treated Patients
• Use the lowest effective dosage for the shortest possible duration.
• Avoid administration of more than one NSAID at a time.
• Avoid use in patients at higher risk, unless benefits are expected to outweigh the increased risk of bleeding. For such patients, as well as those with active GI bleeding, consider alternate therapies other than NSAIDs.
• Remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy.
• If a serious GI adverse event is suspected, promptly initiate evaluation and treatment, and discontinue COMBOGESIC IV until a serious GI adverse event is ruled out.
• In the setting of concomitant use of low-dose aspirin for cardiac prophylaxis, monitor patients more closely for evidence of GI bleeding [see Drug Interactions (7)].
5.5 Hypertension
NSAIDs, including the ibuprofen in COMBOGESIC IV, can lead to onset of new hypertension or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Patients taking angiotensin converting enzyme (ACE) inhibitors, thiazide diuretics, or loop diuretics may have impaired response to these therapies when taking NSAIDs. Monitor blood pressure (BP) during the initiation of NSAID treatment and throughout the course of therapy.
5.6 Heart Failure and Edema
The Coxib and traditional NSAID Trialists’ Collaboration meta-analysis of randomized controlled trials demonstrated an approximately two-fold increase in hospitalizations for heart failure in COX-2 selective-treated patients and nonselective NSAID-treated patients compared to placebo-treated patients. In a Danish National Registry study of patients with heart failure, NSAID use increased the risk of MI, hospitalization for heart failure, and death.
Additionally, fluid retention and edema have been observed in some patients treated with NSAIDs. Use of ibuprofen may blunt the CV effects of several therapeutic agents used to treat these medical conditions (e.g., diuretics, ACE inhibitors, or angiotensin receptor blockers [ARBs]) [see Drug Interactions (7)].
Avoid the use of COMBOGESIC IV in patients with severe heart failure unless the benefits are expected to outweigh the risk of worsening heart failure. If COMBOGESIC IV is used in patients with severe heart failure, monitor patients for signs of worsening heart failure.
5.7 Renal Toxicity and Hyperkalemia
Renal Toxicity
Use of COMBOGESIC IV is not recommended in patients with renal impairment.
Long-term administration of NSAIDs, including the ibuprofen component of COMBOGESIC IV, has resulted in renal papillary necrosis and other renal injury.
Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a NSAID may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, dehydration, hypovolemia, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors or ARBs, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
No information is available from controlled clinical studies regarding the use of COMBOGESIC IV in patients with advanced renal disease. The renal effects of COMBOGESIC IV may hasten the progression of renal dysfunction in patients with pre-existing renal disease.
Correct volume status in dehydrated or hypovolemic patients prior to initiating COMBOGESIC IV.
Hyperkalemia
Increases in serum potassium concentration, including hyperkalemia, have been reported with use of NSAIDs,even in some patients without renal impairment. In patients with normal renal function, these effects have been attributed to a hyporeninemic-hypoaldosteronism state.
5.8 Hypersensitivity and Anaphylactic Reactions
Acetaminophen
There have been post-marketing reports of hypersensitivity and anaphylaxis associated with the use of acetaminophen. Clinical signs included swelling of the face, mouth, and throat, respiratory distress, urticaria, rash, pruritus, and vomiting. There were infrequent reports of life-threatening anaphylaxis requiring emergency medical attention. Discontinue COMBOGESIC IV immediately if symptoms associated with allergy or hypersensitivity occur. Do not use COMBOGESIC IV in patients with acetaminophen allergy.
Ibuprofen
NSAIDS, including the ibuprofen in COMBOGESIC IV, has been associated with anaphylactic reactions in patients with and without known hypersensitivity to ibuprofen and in patients with aspirin-sensitive asthma [see Contraindications (4) and Warnings and Precautions (5.9)]. Discontinue COMBOGESIC IV immediately if symptoms associated with allergy or hypersensitivity occur.
5.9 Exacerbation of Asthma Related to Aspirin Sensitivity
A subpopulation of patients with asthma may have aspirin-sensitive asthma which may include chronic rhinosinusitis complicated by nasal polyps; severe, potentially fatal bronchospasm; and/or intolerance to aspirin and other NSAIDs. Because cross-reactivity between aspirin and other NSAIDs has been reported in such aspirin-sensitive patients, COMBOGESIC IV is contraindicated in patients with this form of aspirin sensitivity [see Contraindications (4)]. When COMBOGESIC IV is used in patients with preexisting asthma
(without known aspirin sensitivity), monitor patients for changes in the signs and symptoms of asthma.
5.10 Serious Skin Reactions
COMBOGESIC IV contains acetaminophen and ibuprofen. Acetaminophen, or NSAIDs, including ibuprofen, may cause serious skin reactions such as exfoliative dermatitis, acute generalized exanthematous pustulosis (AGEP), Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Inform patients about the signs and symptoms of serious skin reactions, and discontinued the use of the drug at the first appearance of skin rash or any other sign of hypersensitivity. COMBOGESIC IV is contraindicated in patients with previous serious skin reactions to acetaminophen or NSAIDs [see Contraindications (4)].
5.11 Drug Rash with Eosinophilia and Systemic Symptoms (DRESS)
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) has been reported in patients taking NSAIDs such as the ibuprofen in COMBOGESIC IV. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling. Other clinical manifestations may include hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis. Sometimes symptoms of DRESS may resemble an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its presentation, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, discontinue COMBOGESIC
IV and evaluate the patient immediately.
5.12 Fetal Toxicity
Premature Closure of Fetal Ductus Arteriosus
Avoid use of NSAID-containing products, including COMBOGESIC IV, in pregnant women at about 30 weeks gestation and later. NSAID-containing products, including COMBOGESIC IV, increase the risk of premature closure of the fetal ductus arteriosus at approximately this gestational age.
Oligohydramnios/Neonatal Renal Impairment
Use of NSAID-containing products, including COMBOGESIC IV, at about 20 weeks gestation or later in pregnancy may cause fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation. Oligohydramnios is often, but not always, reversible with treatment discontinuation. Complications of prolonged oligohydramnios may, for example, include limb contractures and delayed lung maturation. In some post-marketing cases of impaired neonatal renal function, invasive procedures such as exchange transfusion or dialysis were required.
If, after careful consideration of alternative treatment options for pain management, NSAID treatment is necessary between about 20 weeks and 30 weeks gestation, limit COMBOGESIC IV use to the lowest effective dose and shortest duration possible. Consider ultrasound monitoring of amniotic fluid if COMBOGESIC IV treatment extends beyond 48 hours. Discontinue COMBOGESIC IV if oligohydramnios occurs and follow up according to clinical practice [see Use in Specific Populations (8.1)].
5.13 Hematologic Toxicity
Anemia has occurred in NSAID-treated patients. This may be due to occult or gross GI blood loss, fluid retention, or an incompletely described effect on erythropoiesis. If a patient being treated with COMBOGESIC IV has any signs or symptoms of anemia, monitor hemoglobin or hematocrit.
NSAIDs, including the ibuprofen in COMBOGESIC IV, may increase the risk of bleeding events. Co-morbid conditions such as coagulation disorders or concomitant use of warfarin, other anticoagulants, antiplatelet agents (e.g., aspirin), serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) may increase this risk. Monitor these patients for signs of bleeding [see Drug Interactions (7)].
5.14 Ophthalmological Effects
Blurred or diminished vision, scotomata, and/or changes in color vision have been reported with oral ibuprofen. If a patient develops such complaints while receiving COMBOGESIC IV, the drug should be discontinued, and the patient should have an ophthalmologic examination which includes central visual fields and color vision testing.
5.15 Aseptic Meningitis
Aseptic meningitis with fever and coma has been observed in patients on oral ibuprofen. Although it is probably more likely to occur in patients with systemic lupus erythematosus and related connective tissue diseases, it has been reported in patients who do not have underlying chronic disease. If signs or symptoms of meningitis develop in a patient on COMBOGESIC IV, the possibility of its being related to ibuprofen should be considered.
5.16 Masking of Inflammation and Fever
The pharmacological activity of COMBOGESIC IV in reducing inflammation, and possibly fever, may diminish the utility of diagnostic signs in detecting infections.
5.17 Laboratory Monitoring
Because serious GI bleeding, hepatotoxicity, and renal injury can occur without warning symptoms or signs, consider monitoring patients on NSAID treatment with a CBC and a chemistry profile as clinically indicated [see Warnings and Precautions (5.3,5.4,5.8)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions to ibuprofen or acetaminophen are described elsewhere in other sections of the labelling.
• Hepatotoxicity [see Warnings and Precautions (5.2)]
• Cardiovascular Thrombotic Events [see Warnings and Precautions (5.3)]
• Gastrointestinal Bleeding, Ulceration, and Perforation [see Warnings and Precautions (5.4)]
• Hypertension [see Warnings and Precautions (5.5)]
• Heart Failure and Edema [see Warnings and Precautions (5.6)]
• Renal Toxicity and Hyperkalemia [see Warnings and Precautions (5.7)]
• Hypersensitivity and Anaphylactic Reactions [see Warnings and Precautions (5.8)]
• Exacerbation of Asthma Related to Aspirin Sensitivity [see Warnings and Precautions (5.9)]
• Serious Skin Reactions [see Warnings and Precautions (5.10)]
• Drug Rash with Eosinophilia and Systemic Symptoms (DRESS) [see Warnings and Precautions (5.11)]
• Hematologic Toxicity [see Warnings and Precautions (5.13)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The clinical trials of COMBOGESIC IV have been conducted in patients with postoperative musculoskeletal pain and soft tissue pain models lasting between two to five days. Two Phase 3 clinical trials have been conducted with COMBOGESIC IV to assess efficacy and safety after multiple doses. In AFT-MXIV-07 participants were treated with COMBOGESIC IV, acetaminophen IV, ibuprofen IV or placebo for a treatment period of 48 hours. In AFT-MXIV-11 participants were treated for between 48 hours and five days with COMBOGESIC IV. The study population for AFT-MXIV-07 was comprised of adults aged 18 to 65 years, mean age: 42 years. AFT-MXIV-11 included adults aged 19 – 87 years, mean age: 53 years.
Safety data for the first 48 hours of both studies was pooled. Overall, 59.3% of the patients (N = 182/307) administered COMBOGESIC IV experienced one or more treatment-emergent adverse event (TEAE) during the first 48 hours of treatment, accounting for a total of 436 TEAEs (see Table 1). The most common TEAEs were related to the infusion site (infusion site pain, infusion site extravasation), or affected the gastrointestinal (nausea, vomiting, constipation) or nervous (dizziness, headache, somnolence) systems.
Table 1: Common TEAEs (occurring in ≥ 3% of COMBOGESIC IV-treated participants)
| Adverse Reactions | COMBOGESIC IV (N=307) % | Acetaminophen (N=75) % | Ibuprofen (N=76) % | Placebo (N=50) % |
| Gastrointestinal disorders | ||||
| Nausea | 16.3 | 33.3 | 34.2 | 32.0 |
| Vomiting | 6.2 | 14.7 | 6.6 | 2.0 |
| Constipation | 7.2 | 5.3 | 5.3 | 8.0 |
| Infusion Site Complications | ||||
| Infusion site pain | 17.6 | 0.0 | 9.2 | 2.0 |
| Infusion site extravasation | 6.5 | 2.7 | 6.6 | 14.0 |
| Nervous System Disorders | ||||
| Headache | 5.5 | 6.7 | 6.6 | 20.0 |
| Dizziness | 7.2 | 9.3 | 9.2 | 18.0 |
| Somnolence | 3.9 | 8.0 | 7.9 | 6.0 |
Other skin and subcutaneous-related TEAEs (pruritis, hyperhidrosis) also affected around 2-3% of the study population, as did procedural nausea and polyuria.
AFT-MXIV-11 found no notable difference in the safety profile of COMBOGESIC IV in participants treated for 5 days compared to those treated for 48 hours. Additionally, the safety profile was comparable between older participants (aged 65-75 years and >75 years) and younger participants (aged <65 years); the type and incidence of treatment-emergent adverse events was comparable, and the incidence of clinically significant shifts in laboratory tests (hematocrit 1.3% (n=3/228), hemoglobin 1.3% (n=3/228) and erythrocytes 0.9% (n=2/218), was low in participants over the age of 65.
Related/similar drugs
7. Drug Interactions
Table 2. Drug Interactions with COMBOGESIC IV
| Drugs That Interfere with Hemostasis | |
| Clinical Impact: | • Ibuprofen and anticoagulants such as warfarin have a synergistic effect on bleeding. The concomitant use of ibuprofen and anticoagulants have an increased risk of serious bleeding compared to the use of either drug alone. • Serotonin release by platelets plays an important role in hemostasis. Case-control and cohort epidemiological studies showed that concomitant use of drugs that interfere with serotonin reuptake and an NSAID may potentiate the risk of bleeding more than an NSAID alone. |
| Intervention: | Monitor patients with concomitant use of COMBOGESIC IV with anticoagulants (e.g., warfarin), antiplatelet agents (e.g., aspirin), selective serotonin reuptake inhibitors (SSRIs), and serotonin norepinephrine reuptake inhibitors (SNRIs) for signs of bleeding [see Warnings and Precautions (5.13)]. |
| Aspirin | |
| Clinical Impact: | Controlled clinical studies showed that the concomitant use of NSAIDs and analgesic doses of aspirin does not produce any greater therapeutic effect than the use of NSAIDs alone. In a clinical study, the concomitant use of an NSAID and aspirin was associated with a significantly increased incidence of GI adverse reactions as compared to use of the NSAID alone [see Warnings and Precautions (5.3)]. |
| Intervention: | Concomitant use of COMBOGESIC IV and analgesic doses of aspirin is not generally recommended because of the increased risk of bleeding [see Warnings and Precautions (5.4, 5.13)]. COMBOGESIC IV is not a substitute for low dose aspirin for cardiovascular protection. |
| ACE Inhibitors, Angiotensin Receptor Blockers, and Beta-Blockers | |
| Clinical Impact: | • NSAIDs may diminish the antihypertensive effect of angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), or beta-blockers (including propranolol). • In patients who are elderly, volume-depleted (including those on diuretic therapy), or have renal impairment, co-administration of an NSAID with ACE inhibitors or ARBs may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. |
| Intervention: | • During concomitant use of COMBOGESIC IV and ACE-inhibitors, ARBs, or betablockers, monitor blood pressure to ensure that the desired blood pressure is obtained. • During concomitant use of COMBOGESIC IV and ACE-inhibitors or ARBs in patients who are elderly, volume-depleted, or have impaired renal function, monitor for signs of worsening renal function [see Warnings and Precautions (5.7)]. • When these drugs are administered concomitantly, patients should be adequately hydrated. Assess renal function at the beginning of the concomitant treatment and periodically thereafter. |
| Diuretics | |
| Clinical Impact: | Clinical studies, as well as post-marketing observations, showed that NSAIDs reduced the natriuretic effect of loop diuretics (e.g., furosemide) and thiazide diuretics in some patients. This effect has been attributed to the NSAID inhibition of renal prostaglandin synthesis. |
| Intervention: | During concomitant use of COMBOGESIC IV with diuretics, observe patients for signs of worsening renal function, in addition to assuring diuretic efficacy including antihypertensive effects [see Warnings and Precautions (5.7)]. |
| Digoxin | |
| Clinical Impact: | The concomitant use of ibuprofen with digoxin has been reported to increase the serum concentration and prolong the half-life of digoxin. |
| Intervention: | During concomitant use of COMBOGESIC IV and digoxin, monitor serum digoxin levels. |
| Lithium | |
| Clinical Impact: | NSAIDs have produced elevations in plasma lithium levels and reductions in renal lithium clearance. The mean minimum lithium concentration increased 15%, and the renal clearance decreased by approximately 20%. This effect has been attributed to NSAID inhibition of renal prostaglandin synthesis. |
| Intervention: | During concomitant use of COMBOGESIC IV and lithium, monitor patients for signs of lithium toxicity. |
| Methotrexate | |
| Clinical Impact: | Concomitant use of NSAIDs and methotrexate may increase the risk for methotrexate toxicity (e.g., neutropenia, thrombocytopenia, renal dysfunction). |
| Intervention: | During concomitant use of COMBOGESIC IV and methotrexate, monitor patients for methotrexate toxicity. |
| Cyclosporine | |
| Clinical Impact: | Concomitant use of NSAIDS and cyclosporine may increase cyclosporine’s nephrotoxicity. |
| Intervention: | During concomitant use of COMBOGESIC IV and cyclosporine, monitor patients for signs of worsening renal function. |
| NSAIDs and Salicylates | |
| Clinical Impact: | Concomitant use of ibuprofen with other NSAIDs or salicylates (e.g., diflunisal, salsalate) increases the risk of GI toxicity, with little or no increase in efficacy [see Warnings and Precautions (5.4)]. |
| Intervention: | The concomitant use of ibuprofen with other NSAIDs or salicylates is not recommended. |
| Pemetrexed | |
| Clinical Impact: | Concomitant use of NSAIDS and pemetrexed may increase the risk of pemetrexed-associated myelosuppression, renal, and GI toxicity (see the pemetrexed prescribing information). |
| Intervention: | During concomitant use of COMBOGESIC IV and pemetrexed, in patients with renal impairment whose creatinine clearance ranges from 45 to 79 mL/min, monitor for myelosuppression, renal and GI toxicity. NSAIDs with short elimination half-lives (e.g., diclofenac, indomethacin) should be avoided for a period of two days before, the day of, and two days following administration of pemetrexed. In the absence of data regarding potential interaction between pemetrexed and NSAIDs with longer half-lives (e.g., meloxicam, nabumetone), patients taking these NSAIDs should interrupt dosing for at least five days before, the day of, and two days following pemetrexed administration. |
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Use of NSAID-containing products, including COMBOGESIC IV, can cause premature closure of the fetal ductus arteriosus and fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. Because of these risks, limit dose and duration of COMBOGESIC IV use between about 20 and 30 weeks of gestation and avoid COMBOGESIC IV use at about 30 weeks of gestation and later in pregnancy (see Clinical Considerations, Data).
Premature Closure of Fetal Ductus Arteriosus:
Use of NSAID-containing products, including COMBOGESIC IV, at about 30 weeks gestation or later in pregnancy increases the risk of premature closure of the fetal ductus arteriosus.
Oligohydramnios/Neonatal Renal Impairment:
Use of NSAID-containing products, including COMBOGESIC IV, at about 20 weeks gestation or later in pregnancy has been associated with cases of fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment.
Data from observational studies regarding other potential embryofetal risks of NSAID use in women in the first or second trimester of pregnancy are inconclusive.
No adequate and well-controlled studies have been conducted using COMBOGESIC IV in pregnant women. Animal reproduction studies have also not been conducted with COMBOGESIC IV.
The following describes animal reproduction studies for Acetaminophen and Ibuprofen:
Acetaminophen: Reproductive and developmental studies in rats and mice from the published literature have identified adverse events at clinically relevant doses of acetaminophen. Fetotoxicity, increases in bone variations in the fetuses, and necrosis in the fetus liver and kidney have been noted in studies in rats. In mice treated with acetaminophen at doses within the clinical dosing range, cumulative adverse effects on reproduction were seen in a continuous breeding study. A reduction in number of litters of the parental mating pair was observed as well as retarded growth and abnormal sperm in their offspring and reduced birth weight in the next generation (see Data).
Ibuprofen: Reproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities after ibuprofen exposure. However, animal reproduction studies are not always predictive of human response. There are no adequate and well-controlled studies in pregnant women and ibuprofen should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus (see Data).
Based on animal data, prostaglandins have been shown to have an important role in endometrial vascular permeability, blastocyst implantation, and decidualization. In animal studies, administration of prostaglandin synthesis inhibitors such as ibuprofen, resulted in increased pre- and post-implantation loss. Prostaglandins also have been shown to have an important role in fetal kidney development. In published animal studies, prostaglandin synthesis inhibitors have been reported to impair kidney development when administered at clinically relevant doses (see Data).
The estimated background risk of major birth defects and miscarriages for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the general U.S. population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Premature Closure of Fetal Ductus Arteriosus:
Avoid use of NSAID-containing products, including COMBOGESIC IV, in women at about 30 weeks gestation and later in pregnancy, because NSAIDs, including COMBOGESIC IV, can cause premature closure of the fetal ductus arteriosus (see Data).
Oligohydramnios/Neonatal Renal Impairment:
If, after consideration of alternative treatments for pain management, an NSAID-containing product, including COMBOGESIC IV, is necessary at about 20 weeks gestation or later in pregnancy, limit the use to the lowest effective dose and shortest duration possible. If COMBOGESIC IV treatment extends beyond 48 hours, consider monitoring with ultrasound for oligohydramnios. If oligohydramnios occurs, discontinue COMBOGESIC IV and follow up according to clinical practice (see Data).
Labor or Delivery
There are no studies on the effects of COMBOGESIC IV during labor or delivery.
In animal studies, NSAIDS, including ibuprofen, inhibit prostaglandin synthesis, cause delayed parturition, and increase the incidence of stillbirth.
Data
Human Data
Acetaminophen:
The results from a large population-based prospective cohort, including data from 26,424 women with live born singletons who were exposed to oral acetaminophen during the first trimester, indicate no increased risk for congenital malformations, compared to a control group of unexposed children. The rate of congenital malformations (4.3%) was similar to the rate in the general population. A population-based, case-control study from the National Birth Defects Prevention Study showed that 11,610 children with prenatal exposure to acetaminophen during the first trimester had no increased risk of major birth defects compared to 4,500 children in the control group. Other epidemiological data showed similar results. However, these studies cannot definitely establish the absence of any risk because of methodological limitations, including recall bias.
Ibuprofen:
Premature Closure of Fetal Ductus Arteriosus:
Published literature reports that the use of NSAIDs at about 30 weeks of gestation and later in pregnancy may cause premature closure of the fetal ductus arteriosus.
Oligohydramnios/Neonatal Renal Impairment:
Published studies and post-marketing reports describe maternal NSAID use at about 20 weeks gestation or later in pregnancy associated with fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation. In many cases, but not all, the decrease in amniotic fluid was transient and reversible with cessation of the drug. There have been a limited number of case reports of maternal NSAID use and neonatal renal dysfunction without oligohydramnios, some of which were irreversible. Some cases of neonatal renal dysfunction required treatment with invasive procedures, such as exchange transfusion or dialysis.
Methodological limitations of these post-marketing studies and reports include lack of a control group; limited information regarding dose, duration, and timing of drug exposure; and concomitant use of other medications. These limitations preclude establishing a reliable estimate of the risk of adverse fetal and neonatal outcomes with maternal NSAID use. Because the published safety data on neonatal outcomes involved mostly preterm infants, the generalizability of certain reported risks to the full-term infant exposed to NSAIDs through maternal use is uncertain.
Animal Data
Acetaminophen:
Studies in pregnant rats that received oral acetaminophen during organogenesis at doses up to 0.85 times the maximum human daily dose (MHDD= 4 grams/day, based on a body surface area comparison) showed evidence of fetotoxicity (reduced fetal weight and length) and a dose-related increase in bone variations (reduced ossification and rudimentary rib changes). Offspring had no evidence of external, visceral, or skeletal malformations.
When pregnant rats received oral acetaminophen throughout gestation at doses of 1.2 times the MHDD (based on a body surface area comparison), areas of necrosis occurred in both the liver and kidney of pregnant rats and fetuses. These effects did not occur in animals that received oral acetaminophen at doses 0.3 times the MHDD (based on a body surface area comparison).
In a continuous breeding study, pregnant mice received 0.25, 0.5, or 1.0% acetaminophen via the diet (357, 715, or 1430 mg/kg/day). These doses are approximately 0.43, 0.87, and 1.7 times the MHDD, respectively, based on a body surface area comparison. A dose-related reduction in body weights of fourth and fifth litter offspring of the treated mating pair occurred during lactation and post-weaning at all doses. Animals in the high dose group had a reduced number of litters permating pair,male offspring with an increased percentage of abnormal sperm, and reduced birth weights in the next generation pups.
Ibuprofen:
In a published study, female rabbits given 7.5, 20, or 60 mg/kg ibuprofen (0.12, 0.32, or 0.97-times the maximum human daily dose of 1,200 mg of ibuprofen based on a body surface area comparison) from Gestation Days 1 to 29, no clear treatment-related adverse developmental effects were noted. This dose was associated with significant maternal toxicity (stomach ulcers, gastric lesions). In the same publication, female rats were administered 7.5, 20, 60, 180 mg/kg ibuprofen (0.06, 0.16, 0.48, 1.5-times the maximum daily dose) did not result in clear adverse developmental effects. Maternal toxicity (gastrointestinal lesions) was noted at 20 mg/kg and above.
In a published study, rats were orally dosed with 300 mg/kg ibuprofen (2.4-times the maximum human daily dose of 1,200 mg based on a body surface area comparison) during Gestation Days 9 and 10 (critical time points for heart development in rats). Ibuprofen treatment resulted in an increase in the incidence of membranous ventricular septal defects. This dose was associated with significant maternal toxicity including gastrointestinal toxicity. One incidence each of a membranous ventricular septal defect and gastroschisis was noted fetuses from rabbits treated with 500 mg/kg (8.1-times the maximum human daily dose) from Gestation Day 9 to 11.
8.2 Lactation
Risk Summary
The components of COMBOGESIC IV, ibuprofen and acetaminophen, are present in human milk. Limited published literature reports that, orally administered ibuprofen is present in human milk at relative infant doses of 0.06% to 0.6% of the maternal weight-adjusted daily dose. There are no reports of adverse effects of ibuprofen on the breastfed infant and no effects on milk production.
Limited published studies report that orally administered acetaminophen passes rapidly into human milk with similar levels in the milk and plasma. Average and maximum neonatal doses of 1% and 2%, respectively, of the weight-adjusted maternal dose are reported after a single oral administration of 1 gram acetaminophen. There is one well-documented report of a rash in a breast-fed infant that resolved when the mother stopped acetaminophen use and recurred when she resumed acetaminophen use.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for COMBOGESIC IV and any potential adverse effects on the breastfed infant from COMBOGESIC IV or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Infertility
Acetaminophen
Based on animal data, use of acetaminophen may cause reduced fertility in males and females of reproductive potential. It is not known whether these effects on fertility are reversible.
Published studies in rodents report that oral acetaminophen treatment of male animals at doses that are approximately 1.2 times the MHDD and greater (based on a body surface area comparison) result in decreased testicular weights, reduced spermatogenesis, and reduced fertility. In female animals given the same doses, reduced implantation sites were reported. Additional published animal studies indicate that acetaminophen exposure in utero adversely impacts reproductive capacity of both male and female offspring at clinically relevant exposures [see Nonclinical Toxicology (13.1)].
Ibuprofen
Based on the mechanism of action, the use of prostaglandin-mediated NSAID-containing products, including COMBOGESIC IV, may delay or prevent rupture of ovarian follicles, which has been associated with reversible infertility in some women. Published animal studies have shown that administration of prostaglandin synthesis inhibitors has the potential to disrupt prostaglandin mediated follicular rupture required for ovulation. Small studies in women treated with NSAIDs have also shown a reversible delay in ovulation. Consider withdrawal of NSAID-containing products, including COMBOGESIC IV, in women who have difficulties conceiving or who are undergoing investigation of infertility.
8.4 Pediatric Use
The safety and effectiveness of COMBOGESIC IV in pediatric patients has not been studied in the pediatric population. COMBOGESIC IV is not approved for patients under 18 years of age.
8.5 Geriatric Use
Of the total number of subjects in clinical studies of COMBOGESIC IV, 20.2% (N = 62/307) were aged 65 years or over, including 5.2% (N = 16/307) aged 75 years or over [see Adverse Reactions (6.1)]. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Elderly patients, compared to younger patients, are at greater risk for NSAID-associated serious cardiovascular, gastrointestinal, and/or renal adverse reactions. If the anticipated benefit for the elderly patient outweighs these potential risks, start dosing at the low end of the dosing range, and monitor patients for adverse effects [see Warnings and Precautions (5.2, 5.3, 5.4, 5.7)].
The ibuprofen and acetaminophen in COMBOGESIC IV are known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Hepatic Impairment
COMBOGESIC IV has not been studied in patients with impaired hepatic function. Because acetaminophen is extensively metabolized by the liver, COMBOGESIC IV is contraindicated in patients with severe hepatic impairment or severe active liver disease. Use of COMBOGESIC IV in patients with hepatic impairment is not recommended [see Warnings and Precautions (5.2)].
8.7 Renal Impairment
COMBOGESIC IV has not been studied in patients with impaired renal function. The use of COMBOGESIC IV in these patients is not recommended [see Warnings and Precautions (5.7)].
10. Overdosage
COMBOGESIC IV is a combination product. The clinical presentation of overdose may include the signs and symptoms of acetaminophen toxicity, ibuprofen toxicity, or both.
Acetaminophen
The initial symptoms seen within the first 24 hours following an acetaminophen overdose are: anorexia, nausea, vomiting, malaise, pallor and diaphoresis.
In acute acetaminophen overdosage, dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma, and thrombocytopenia also occur. Plasma acetaminophen levels > 300 mcg/mL at 4 hours after oral ingestion were associated with hepatic damage in 90% of patients; minimal hepatic damage is anticipated if plasma levels at 4 hours are < 150 mcg/mL or < 37.5 mcg/mL at 12 hours after ingestion [see Warnings and Precautions (5.2)].
Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis, and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion.
If an acetaminophen overdose is suspected, obtain a serum acetaminophen assay as soon as possible, but no sooner than 4 hours following oral ingestion. Obtain liver function studies initially and repeat at 24-hour intervals. Administer the antidote N-acetylcysteine (NAC) as early as possible. As a guide to treatment of acute ingestion, the acetaminophen level can be plotted against time since oral ingestion on a nomogram (Rumack- Matthew). The lower toxic line on the nomogram is equivalent to 150 mcg/mL at 4 hours and 37.5 mcg/mL at 12 hours. If serum level is above the lower line, administer the entire course of NAC treatment. Withhold NAC therapy if the acetaminophen level is below the lower line.
Ibuprofen
Symptoms following acute NSAID overdosages have been typically limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which have been generally reversible with supportive care. Gastrointestinal bleeding has occurred. Hypertension, acute renal failure, respiratory depression, and coma have occurred, but were rare [see Warnings and Precautions (5.4, 5.5, 5.7)].
Manage patients with symptomatic and supportive care following an NSAID overdosage. There are no specific antidotes.
For additional information about overdosage treatment contact a poison control center (1-800-222-1222).
11. Combogesic IV Description
COMBOGESIC IV (acetaminophen and ibuprofen) injection contains acetaminophen and ibuprofen, a nonsteroidal anti-inflammatory drug.
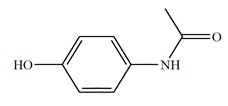
Acetaminophen chemical name is N-acetyl-p-aminophenol. Acetaminophen is a white, odorless, crystalline powder, possessing a slightly bitter taste. Acetaminophen is soluble in boiling water and 1N sodium hydroxide and is freely soluble in alcohol. Acetaminophen has a molecular weight of 151.16. The molecular formula is C8H9NO2 and the structural formula is:
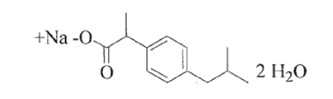
Ibuprofen sodium dihydrate chemical name is 2-(4-isobutyl phenyl) propionic acid sodium salt dihydrate. Ibuprofen sodium dihydrate is a white powder. It has a molecular weight of 264.29. It is freely soluble in water. The molecular formula is C13H21NaO4 and the structural formula of ibuprofen sodium dihydrate is represented below:
COMBOGESIC IV injection is a sterile, clear, colorless, non-pyrogenic, isotonic solution, intended for intravenous infusion with a pH stability range of 6.3-7.3.
Each single-dose 100 mL vial contains 1,000 mg of acetaminophen and 300 mg of ibuprofen base (equivalent to 385 mg of ibuprofen sodium dihydrate), 25 mg of Cysteine hydrochloride monohydrate, 13 mg of Disodium phosphate dihydrate, 3,285 mg of Mannitol, Hydrochloric acid (for pH adjustment), Sodium hydroxide (for pH adjustment), Water for injection.
12. Combogesic IV - Clinical Pharmacology
12.1 Mechanism of Action
COMBOGESIC IV contains acetaminophen and ibuprofen as active drug substances.
Acetaminophen is a non-opiate, non-salicylate analgesic. The precise mechanism of the analgesic properties of acetaminophen is not established but is thought to primarily involve central actions. Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID). Its mechanism of action for analgesia, like that of other NSAIDs, is not completely understood, but involves inhibition of cyclooxygenase (COX-1 and COX-2).
Ibuprofen is a potent inhibitor of prostaglandin synthesis in vitro. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models. Prostaglandins are mediators of inflammation. Because ibuprofen is an inhibitor of prostaglandin synthesis, its mode of action may be due to a decrease of prostaglandins in peripheral tissues.
12.2 Pharmacodynamics
Hematological Effects
NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible.
12.3 Pharmacokinetics
The pharmacokinetic profile of the intravenous formulation is dose proportional following the administration of a half dose and a full dose of COMBOGESIC IV.
The maximum concentration (Cmax) occurs at the end of the 15-min intravenous infusion of COMBOGESIC IV. While overall exposures (area under the concentration time curve [AUC]) were similar following a single dose of COMBOGESIC IV compared to the same dose given orally, the Cmax of the intravenous formulation was twice that of the oral formulation. As expected, the Tmax following intravenous administration was achieved much faster (in 15 minutes) than with the oral formulation. The mean Cmax and AUC0-inf of COMBOGESIC IV following administration of a single intravenous dose of 1,000 mg acetaminophen and 300 mg ibuprofen in adults were 34.30 mcg/mL and 56.48 mcg.h/mL for acetaminophen and 48.12 mcg/mL and 102.82 mcg.h/mL for ibuprofen, respectively.
A single-dose pharmacokinetic study of COMBOGESIC IV in healthy volunteers showed no drug interactions between acetaminophen and ibuprofen.
Distribution
Acetaminophen appears to be widely distributed throughout most body tissues except fat. Its apparent volume of distribution is about 0.9 L/kg. A relative small portion (~20%) of acetaminophen is bound to plasma protein.
Elimination
The half-life of acetaminophen is about 2 to 3 hours in adults. It is somewhat shorter in children and somewhat longer in neonates and in cirrhotic patients. Acetaminophen is eliminated from the body primarily by formation of glucuronide and sulfate conjugates in a dose-dependent manner. Ibuprofen is rapidly metabolized and eliminated in the urine. The serum half-life is 1.8 to 2.0 hours.
Metabolism
Acetaminophen is primarily metabolized in the liver by first-order kinetics and involves three principal separate pathways:
a) conjugation with glucuronide;
b) conjugation with sulfate; and
c) oxidation via the cytochrome, P450-dependent, mixed-function oxidase enzyme pathway to form a reactive intermediate metabolite, which conjugates with glutathione and is then further metabolized to form cysteine and mercapturic acid conjugates. The principal cytochrome P450 isoenzyme involved appears to be CYP2E1, with CYP1A2 and CYP3A4 as additional pathways.
In adults, the majority of acetaminophen is conjugated with glucuronic acid and, to a lesser extent, with sulfate. These glucuronide-, sulfate-, and glutathione-derived metabolites lack biologic activity. In premature infants, newborns, and young infants, the sulfate conjugate predominates.
Excretion
Less than 9% of acetaminophen is excreted unchanged in the urine.
The excretion of ibuprofen is virtually complete 24 hours after the last dose.
Studies have shown that following ingestion of the drug, 45% to 79% of the dose was recovered in the urine within 24 hours as metabolite A (25%), (+)-2-[p-(2hydroxymethyl-propyl) phenyl] propionic acid and metabolite B (37%), (+)-2-[p-(2carboxypropyl)phenyl]propionic acid; the percentages of free and conjugated ibuprofen were approximately 1% and 14%, respectively.
Specific Populations
Pediatric Patients
The pharmacokinetics of COMBOGESIC IV has not been studied in pediatric patients below 18 years of age.
Hepatic Impairment
The pharmacokinetics of COMBOGESIC IV in patients with impaired hepatic function has not been studied [see Warnings and Precautions (5.2) and Use in Specific Populations (8.6)].
Renal Impairment
The pharmacokinetics of COMBOGESIC IV in patients with renal impairment has not been studied. [see Warnings and Precautions (5.7) and Use in Specific Populations (8.7)].
Drug Interaction Studies
Aspirin: When NSAIDs were administered with aspirin, the protein binding of NSAIDs were reduced, although the clearance of free NSAID was not altered. The clinical significance of this interaction is not known. See Table 2 for clinically significant drug interactions of NSAIDs with aspirin [see Drug Interactions (7)].
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Acetaminophen
Long-term studies in mice and rats have been completed by the National Toxicology Program to evaluate the carcinogenic potential of acetaminophen. In 2-year feeding studies, F344/N rats and B6C3F1 mice were fed a diet containing acetaminophen up to 6000 ppm. Female rats demonstrated equivocal evidence of carcinogenic activity based on increased incidences of mononuclear cell leukemia at 0.8 times the MHDD (based on a body surface area comparison). In contrast, there was no evidence of carcinogenic activity in male rats (0.7 times) or mice (1.2 - 1.4 times the MHDD, based on a body surface area comparison).
Ibuprofen
Adequate long-term animal studies have not been conducted to evaluate the carcinogenic potential of ibuprofen.
Mutagenesis
Acetaminophen
Acetaminophen was not mutagenic in the bacterial reverse mutation assay (Ames test). In contrast, acetaminophen tested positive in the in vitro mouse lymphoma assay and the in vitro chromosomal aberration assay using human lymphocytes.
In the published literature, acetaminophen has been reported to be clastogenic when administered a dose of 1,500 mg/kg/day to the rat model (at 3.6 times the MHDD, based on a body surface area comparison). In contrast, no clastogenicity was noted at a dose of 750 mg/kg/day (1.8 times the MHDD, based on a body surface area comparison), suggesting a threshold effect.
Ibuprofen
In published studies, ibuprofen was not mutagenic in the in vitro bacterial reverse mutation assay (Ames assay).
Impairment of Fertility
Acetaminophen
In studies conducted by the National Toxicology Program, fertility assessments with acetaminophen have been completed in Swiss mice via a continuous breeding study. There were no effects on fertility parameters in mice consuming up to approximately 1.1 times the MHDD of acetaminophen, (based on a body surface area comparison). Although there was no effect on sperm motility or sperm density in the epididymis, there was a significant increase in the percentage of abnormal sperm in mice consuming approximately 1.1 times the MHDD (based on a body surface area comparison) and there was a reduction in the number of mating pairs producing a fifth litter at this dose, suggesting the potential for cumulative toxicity with chronic administration of acetaminophen near the upper limit of daily dosing.
Published studies in rodents report that oral acetaminophen treatment of male animals at doses that are approximately 0.8 times the MHDD and greater (based on a body surface area comparison) result in decreased testicular weights, reduced spermatogenesis, and reduced fertility. Females given the same doses also showed reduced implantation sites. These effects appear to increase with the duration of treatment.
Published studies in rodents report that oral acetaminophen treatment of male animals at doses that are 1.2 times the MHDD and greater (based on a body surface area comparison) result in decreased testicular weights, reduced spermatogenesis, reduced fertility, and reduced implantation sites in females given the same doses. These effects appear to increase with the duration of treatment.
In a published mouse study, oral administration of 50 mg/kg acetaminophen to pregnant mice from Gestation Day 7 to delivery (0.06 times the MHDD) reduced the number of primordial follicles in female offspring and reduced the percentage of full-term pregnancies and number of pups born to these females exposed to acetaminophen in utero.
In a published study, pregnant rats oral administration of 350 mg/kg acetaminophen (0.85 times the MHDD) from Gestation Day 13 to 21 (dams), reduced the number of germ cells in the fetal ovary and decreased ovary weight and reduced number of pups per litter in F1 females as well as reduced ovary weights in F2 females.
Ibuprofen
In a published study, dietary administration of ibuprofen to male and female rats 8-weeks prior to and during mating at dose levels of 20 mg/kg (0.16-times the MRHD based on body surface area comparison) did not impact male or female fertility or litter size.
In other studies, adult mice were administered ibuprofen intraperitoneally at a dose of 5.6 mg/kg/day (0.023-times the MRHD based on body surface area comparison) for 35 or 60 days in males and 35 days in females. There was no effect on sperm motility or viability in males but decreased ovulation was reported in females.
14. Clinical Studies
14.1 Phase 3 Clinical Efficacy Study
COMBOGESIC IV was studied in a Phase 3, placebo-controlled, prospective, randomized, double-blind, parallel-design trial comparing the analgesic efficacy and safety of COMBOGESIC IV (n=75/276) with acetaminophen alone (n=75/276), ibuprofen alone (n=76/276) and placebo (n=50/276), after bunionectomy surgery. The demographic and baseline characteristics of the 276 eligible patients were balanced between the treatment groups with the majority of patients being female (82%) and white (62%) with a mean (SD) age of 42.4 (12.2) years.
The primary efficacy endpoint was the time-adjusted Sum of Pain Intensity Differences over 48 hours (SPID48) and analyzed with each pre-rescue Visual Analogue Scale (VAS) carried forward up to 2 hours. An analysis of covariance was used for the primary efficacy analysis with treatment as the fixed effect and baseline pain intensity score as the covariate on the intent to treat population.
The analysis of time-adjusted SPID48 demonstrated that COMBOGESIC IV (least square mean (LSM) = 36.7, standard error (SE) = 2.2) provided more effective pain relief than placebo (LSM = 17.5, SE = 2.7), acetaminophen (LSM = 19.3, SE = 2.2) or ibuprofen (LSM = 24.6, SE = 2.2).
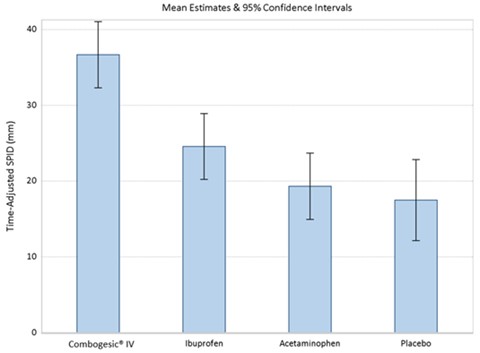
Figure 1: Time-adjusted SPID48 with Pre-Rescue VAS Score Carried Forward up to 2 Hours
16. How is Combogesic IV supplied
COMBOGESIC IV (acetaminophen/ ibuprofen) injection 1,000 mg/300 mg per 100 mL (10 mg/3mg per mL): clear, colorless solution in single-dose vial. Discard unused portion.
NDC # 0143-9150-10: pack of 10 vials.
COMBOGESIC IV is a clear, colorless solution, free from visible particles.
Store at 20°C to 25°C (68°F to 77°F). Excursions permitted between 15°C to 30°C (59°F to 86°F) [See USP
Controlled Room Temperature].
Do not refrigerate or freeze. Store in the original carton in order to protect from light. Protect from heat.
17. Patient Counseling Information
Patients should be informed of the following information before initiating therapy with COMBOGESIC IV.
• Hepatotoxicity: Advise patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness and "flu-like" symptoms). Advise patients to seek immediate medical assistance if these occur [see Warnings and Precautions (5.2)].
• Alcohol: Advise patients that COMBOGESIC IV should not be taken concomitantly with alcohol-containing beverages or other acetaminophen-containing products [see Warnings and Precautions (5.2)].
• Cardiovascular Thrombotic Events: Inform patients that COMBOGESIC IV, like other NSAID-containing medications, may cause serious CV side effects such as MI or stroke, which may result in hospitalization and even death. Advise patients to be alert for the signs and symptoms of cardiovascular thrombotic events including chest pain, shortness of breath, weakness, slurring of speech, and to report any of these symptoms to their health care provider immediately [see Warnings and Precautions (5.3)].
• Gastrointestinal Bleeding, Ulceration, and Perforation: Inform patients that COMBOGESIC IV, like other NSAID-containing medications, can cause GI discomfort and, rarely, serious GI side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Advise patients to be alert for the signs and symptoms of ulcerations and bleeding, including epigastric pain, dyspepsia, melena, and hematemesis. In the setting of concomitant use of low-dose aspirin for cardiac prophylaxis, inform patients of the increased risk for and the signs and symptoms of GI bleeding [see Warnings and Precautions (5.4)].
• Heart Failure and Edema: Advise patients to be alert for the symptoms of congestive heart failure including shortness of breath, unexplained weight gain, or edema and to contact their healthcare provider if such symptoms occur [see Warnings and Precautions (5.6)].
• Weight Gain and Edema: Advise patients to promptly report unexplained weight gain or edema to their physicians [see Warnings and Precautions (5.6)].
• Hypersensitivity and Anaphylactic Reactions: Discontinue COMBOGESIC IV immediately if symptoms associated with allergy or hypersensitivity occur. Do not use COMBOGESIC IV in patients with known acetaminophen or ibuprofen allergy [see Warnings and Precautions (5.8 and 5.10)].
• Serious Skin Reactions, including DRESS: Advise patients to be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching, and to ask for medical advice when observing any indicative sign or symptoms [see Warnings and Precautions (5.8, 5.10, 5.11)].
• Female Fertility: Advise females of reproductive potential who desire pregnancy that NSAID containing products, including COMBOGESIC IV, may be associated with a reversible delay in ovulation [see Use in Specific Populations (8.3)].
• Fetal Toxicity: Inform pregnant women to avoid use of COMBOGESIC IV and other NSAIDs starting at 30 weeks gestation because of the risk of the premature closing of the fetal ductus arteriosus. If treatment with COMBOGESIC IV is needed for a pregnant woman between about 20 to 30 weeks gestation, advise her that she may need to be monitored for oligohydramnios, if treatment continues for longer than 48 hours [see Warnings and Precautions (5.12) and Use in Specific Populations (8.1)].
• Use of NSAIDs and Low-Dose Aspirin: Inform patients not to use low-dose aspirin concomitantly with COMBOGESIC IV until they talk to their healthcare provider [see Warnings and Precautions (5.4), see Drug Interactions (7)].
Manufactured by:
S.M. Farmaceutici SRL, Zona Industriale, 85050 Tito (PZ), Italy
Distributed by: Hikma Pharmaceuticals USA Inc.
Berkeley Heights, NJ 07922
Information and patents: https://www.combogesiciv.com
Revised: April 2024
PRINCIPAL DISPLAY PANEL
NDC 0143-9150-01 Rx only
KEEP OUT OF REACH OF CHILDREN
COMBOGESIC IV
(acetaminophen and ibuprofen) injection
1,000 mg/300 mg per 100 mL (10 mg/3 mg per mL)
For Intravenous Infusion Only
100 mL Single-Dose Vial. Discard unused portion.
NDC 0143-9150-10 Rx only
KEEP OUT OF REACH OF CHILDREN
COMBOGESIC IV
(acetaminophen and ibuprofen) injection
1,000 mg/300 mg per 100 mL (10 mg/3 mg per mL)
For Intravenous Infusion Only
Single-Dose Vial. Discard unused portion.
10 x 100 mL Vials

| COMBOGESIC IV
acetaminophen and ibuprofen injection injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hikma Pharmaceuticals USA Inc. (001230762) |
More about Combogesic IV (acetaminophen / ibuprofen)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- FDA approval history
- Drug class: analgesic combinations
- En español

