AZO Urinary Tract Health: Package Insert / Prescribing Info
Package insert / product label
Generic name: phenazopyridine hydrochloride
Dosage form: tablet
Drug class: Miscellaneous genitourinary tract agents
Medically reviewed by Drugs.com. Last updated on Jun 16, 2025.
On This Page
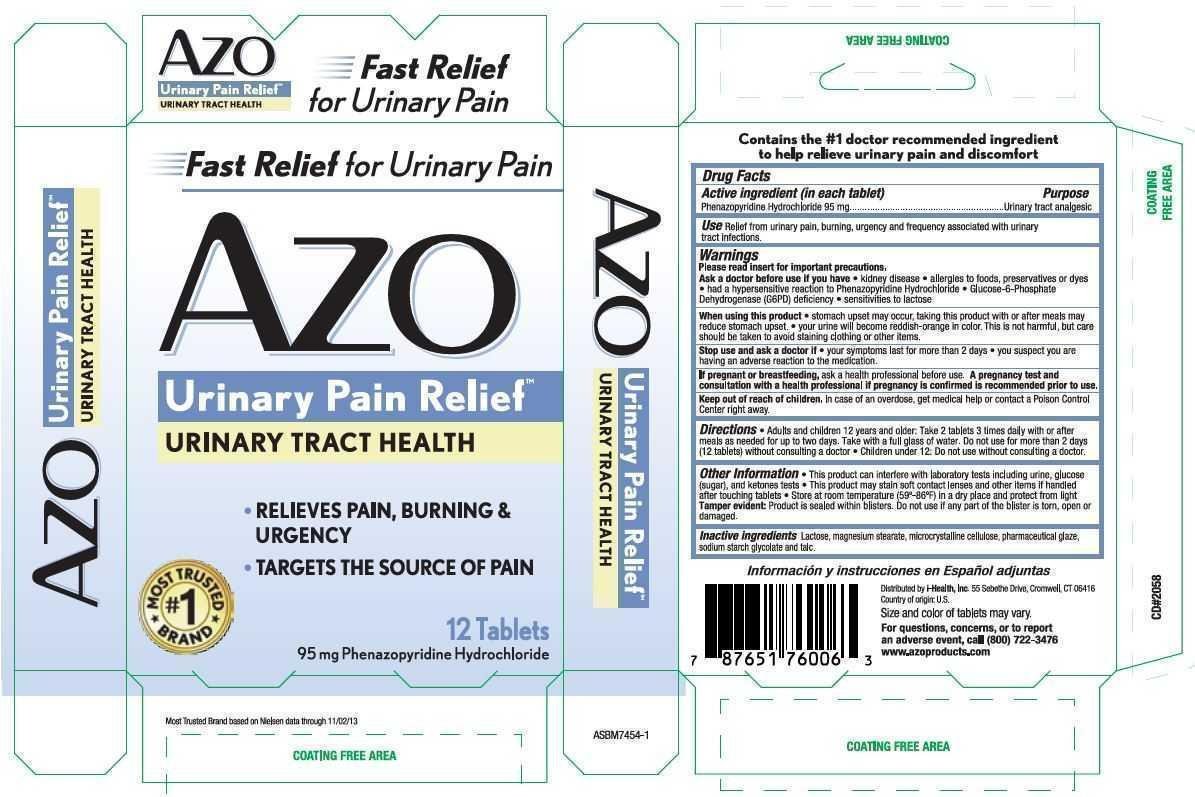
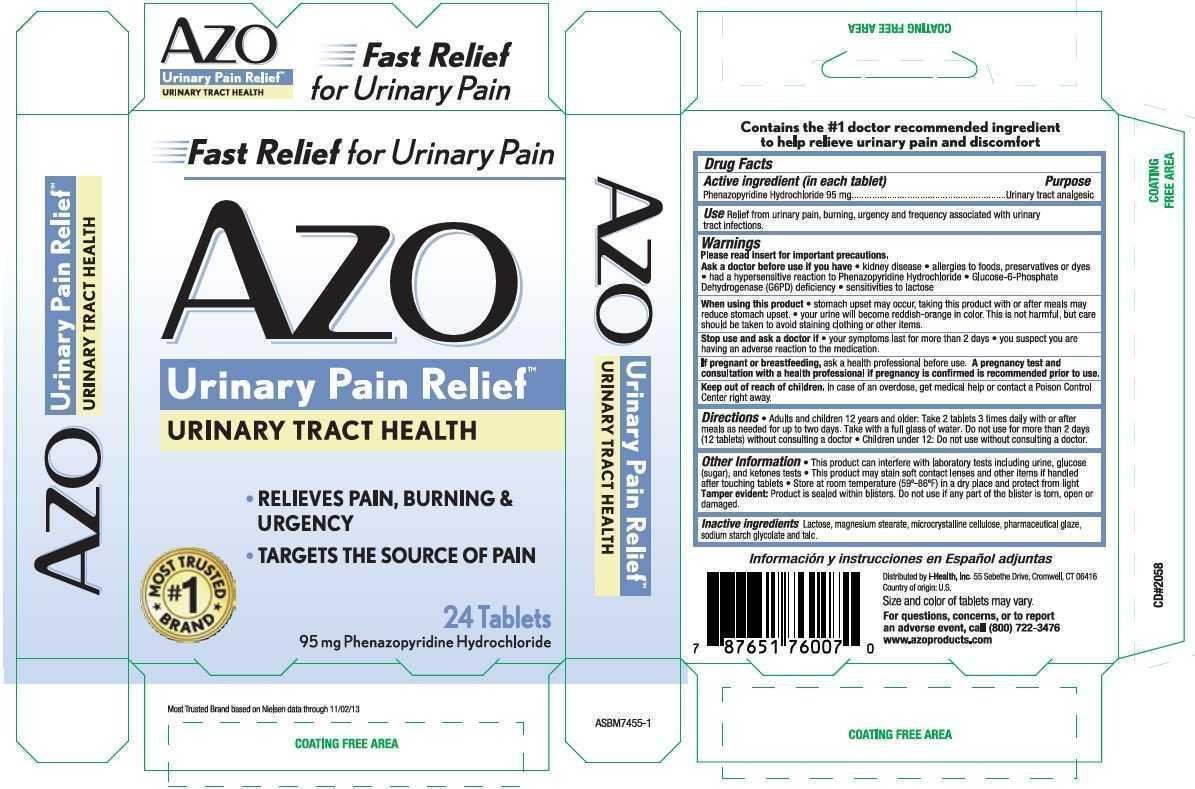
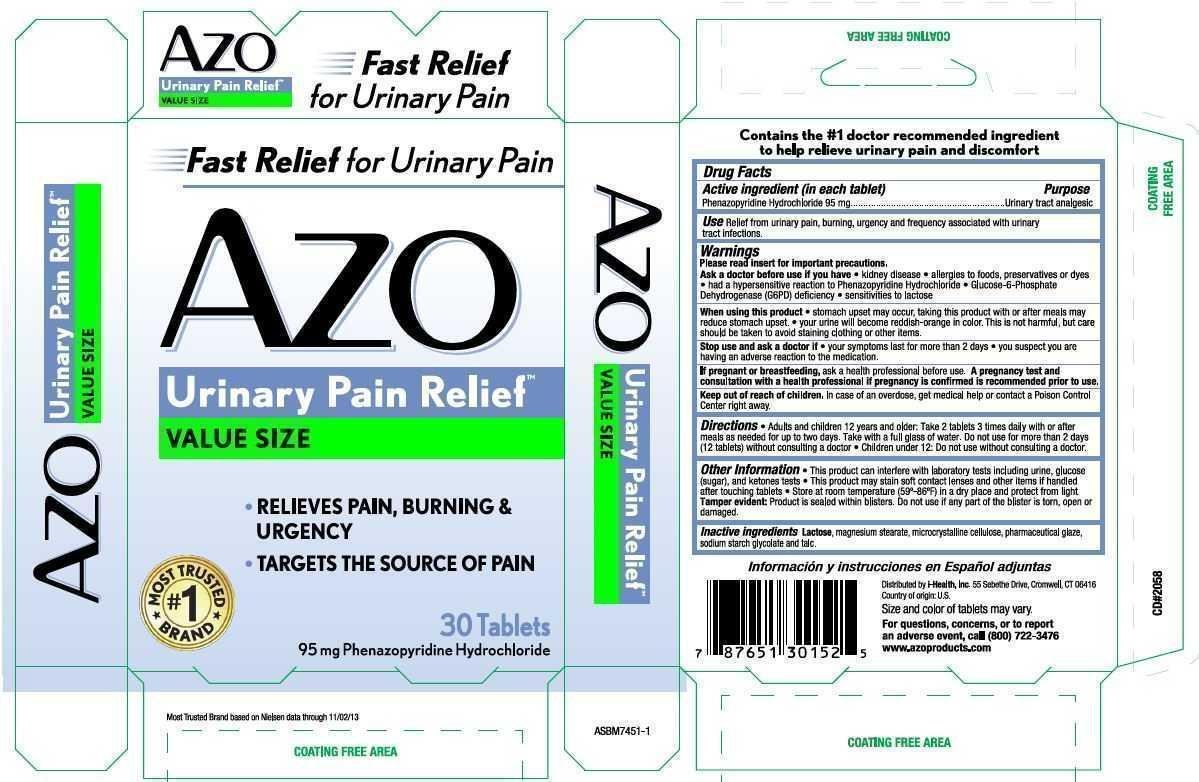
Use Relief from urinary pain, burning, urgency and frequency associated with urinary tract infections.
Warnings
Please read insert for important precautions.
Ask a doctor before use if you have
- kidney disease
- allergies to foods, preservatives or dyes
- had a hypersensitive reaction to Phenazopyridine Hydrochloride
- Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency
- sensitivities to lactose
When using this product
- stomach upset may occur, taking this product with or after meals may reduce stomach upset.
- your urine will become reddish-orange in color. This is not harmful, but care should be taken to avoid staining clothing or other items.
Keep out of reach of children. In case of an overdose, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 12 years and older: Take 2 tablets 3 times daily with or after meals as needed for up to two days. Take with a full glass of water. Do not use for more than 2 days (12 tablets) without consulting a doctor.
- Children under 12: Do not use without consulting a doctor.
Other Information
- This product can interfere with laboratory tests including urine, glucose (sugar), and ketones tests
- This product may stain soft contact lenses and other items if handled after touching tablets
- Store at room temperature (59-86 degrees F) in a dry place and protect from light.
Tamper evident: Product is dealed within blisters. Do not use if any part of the blister is torn, open or damaged.
Inactive ingredients Lactose, magnesium stearate, microcrystalline cellulose, pharmaceutical glaze, sodium starch glycolate and talc.
2 Solutions for Urinary Tract Health*
AZO
Urinary Tract Health
SUPPORT PACK
Urinary Pain Relief
- RELIEVES PAIN, BURNING AND URGENCY
- TARGETS THE SOURCE OF PAIN
MOST TRUSED #1 BRAND
18 Tablets
95 mg Phenazopyridine Hydrochloride
Cranberry
- HELPS FLUSH TO MAINTAIN URINARY TRACT CLEANLINESS*
- WITH PROBIOTIC
14 Caplets
Dietary Supplement
*THESE STATEMENTS HAVE NOT BEEN EVALUATED BY THE FDA. THIS IF NOT INTENDED TO DIAGNOSE, TREAT, CURE OR PREVENT ANY DISEASE.

| AZO URINARY TRACT HEALTH
phenazopyridine hydrochloride tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - i-Health, Inc. (061427694) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Contract Pharmacal Corporation | 057795122 | manufacture(49973-760) | |
Related/similar drugs
Frequently asked questions
More about phenazopyridine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (191)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Support group
- Drug class: miscellaneous genitourinary tract agents
- Breastfeeding

