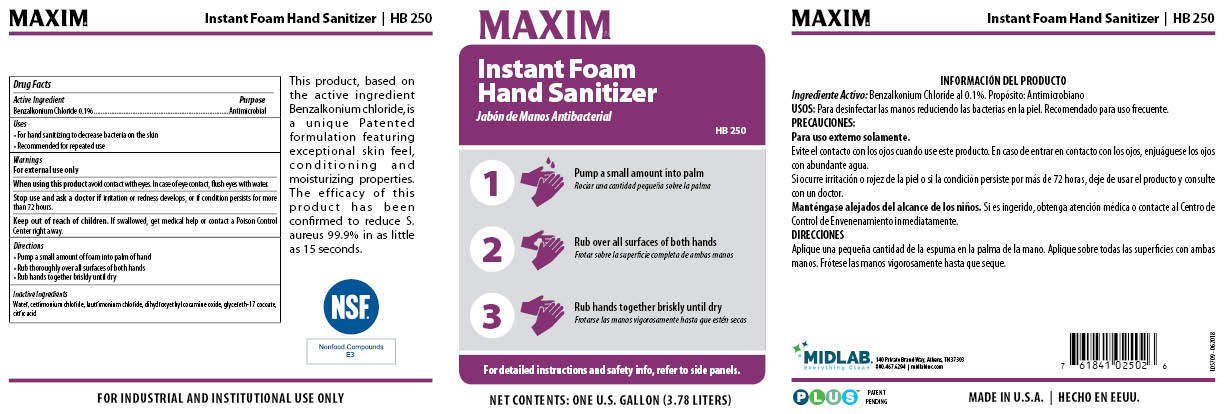
Maxim Instant Foam Hand Sanitizer
Dosage form: soap
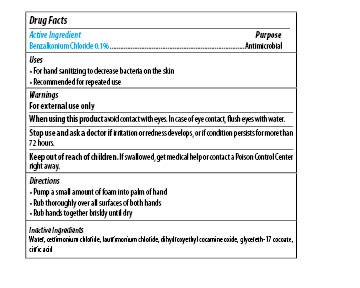
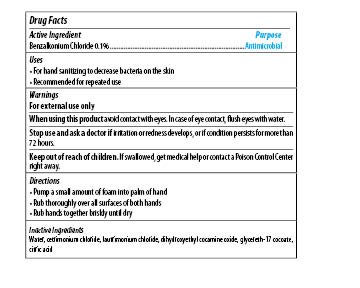
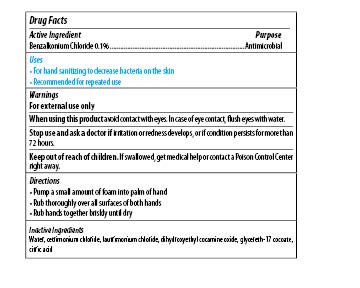
Ingredients: BENZALKONIUM CHLORIDE 0.1mg in 1mL
Labeler: Midlab Incoporated
NDC code: 70542-501
Medically reviewed by Drugs.com. Last updated on May 5, 2025.
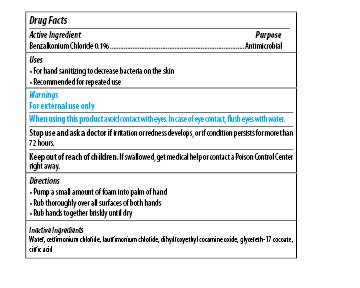
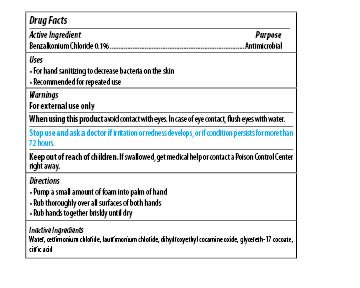
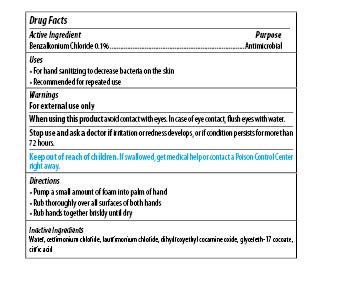
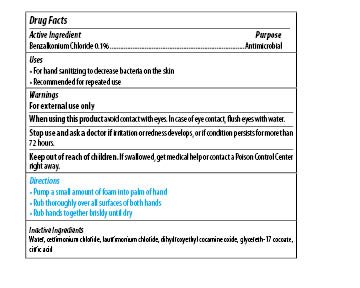
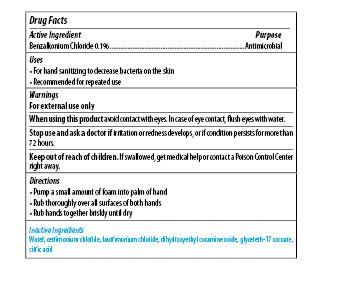
| MAXIM INSTANT FOAM HAND SANITIZER
benzalkonium chloride soap |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Midlab Incoporated (047371463) |
| Registrant - Midlab Incorporated (047371463) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Midlab Incorporated | 047371463 | manufacture(70542-501) | |
Document Id: a59d15ea-d337-2879-e053-2995a90ad9e0
Set id: 54103c39-8993-09e6-e054-00144ff8d46c
Version: 5
Midlab Incoporated
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
