Circle K Eye Drops Redness Reliever
Dosage form: solution/ drops
Ingredients: Tetrahydrozoline Hydrochloride 0.5mg in 1mL
Labeler: Lil Drug Store Products, Inc
NDC code: 66715-5729
Medically reviewed by Drugs.com. Last updated on Feb 28, 2025.
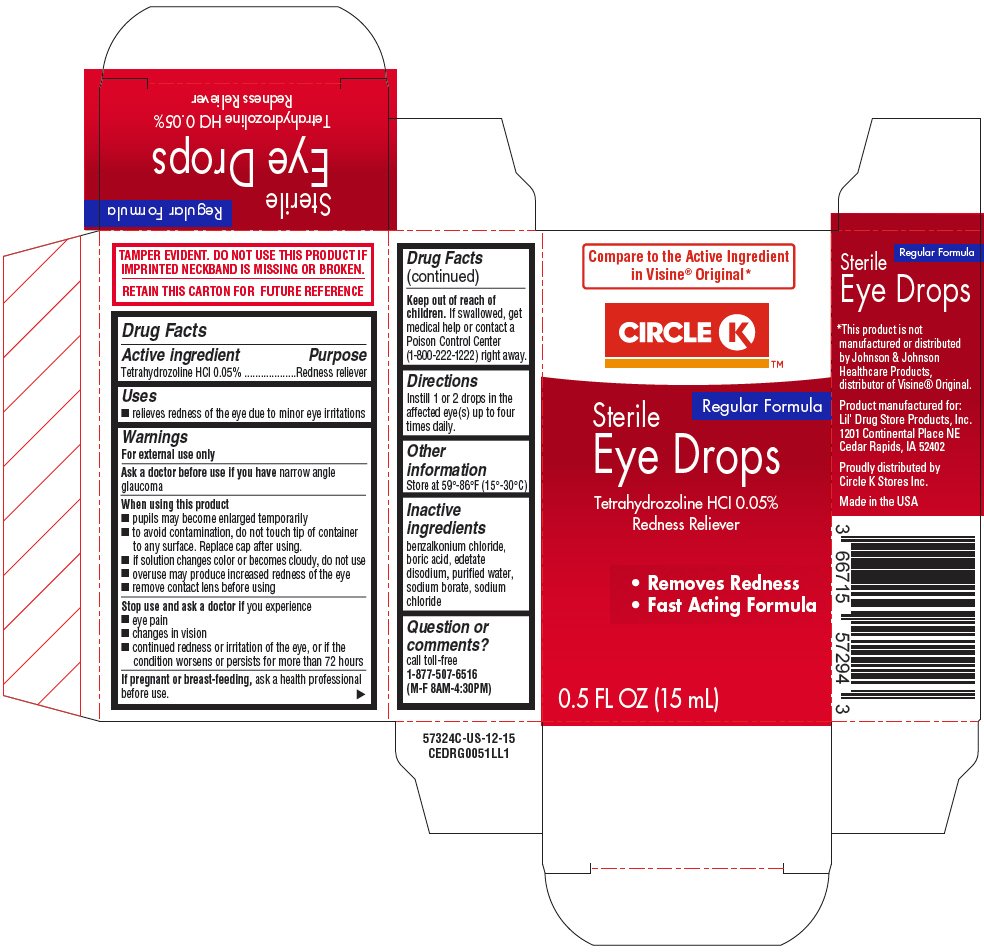
Drug Facts
Tetrahydrozoline HCl 0.05%
Redness reliever
- relieves redness of the eye due to minor eye irritations
For external use only
Ask a doctor before use if you have narrow angle glaucoma
- pupils may become enlarged temporarily
- to avoid contamination, do not touch tip of container to any surface. Replace cap after using.
- if solution changes color or becomes cloudy, do not use
- overuse may produce increased redness of the eye
- remove contact lens before using
Stop use and ask a doctor if you experience
- eye pain
- changes in vision
- continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Instill 1 or 2 drops in the affected eye(s) up to four times daily.
Store at 59°-86°F (15°-30°C)
benzalkonium chloride, boric acid, edetate disodium, purified water, sodium borate, sodium chloride
call toll-free 1-877-507-6516 (M-F 8AM-4:30PM)
Proudly distributed by
Circle K Stores Inc.
Compare to the Active Ingredient
in Visine® Original*
CIRCLE K™
Regular Formula
Sterile
Eye Drops
Tetrahydrozoline HCl 0.05%
Redness Reliever
- Removes Redness
- Fast Acting Formula
0.5 FL OZ (15 mL)
| CIRCLE K EYE DROPS
REDNESS RELIEVER
tetrahydrozoline hydrochloride solution/ drops |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Lil Drug Store Products, Inc (093103646) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
