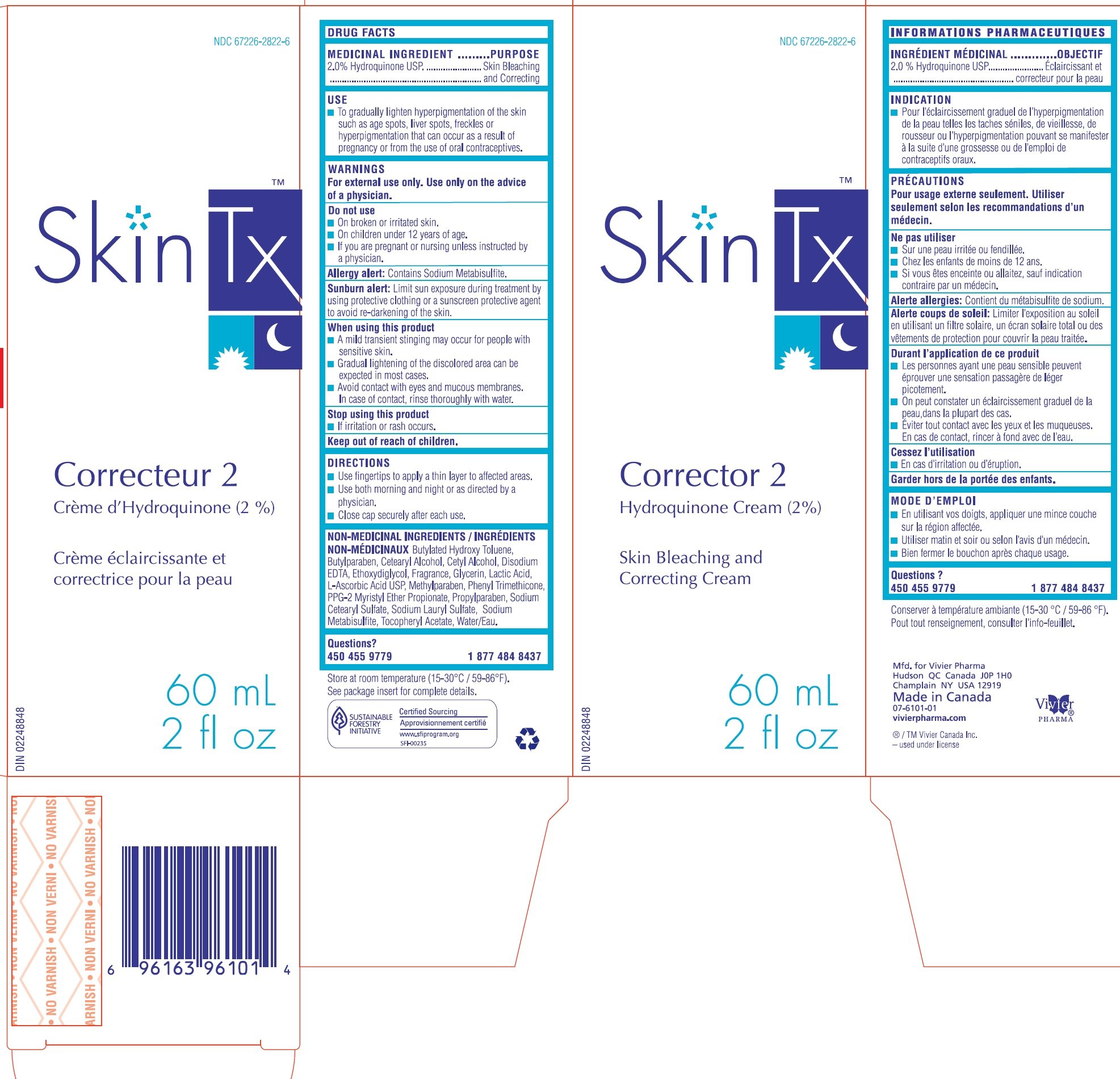
CORRECTOR 2
Dosage form: cream
Ingredients: HYDROQUINONE 2g in 100mL
Labeler: Vivier Pharma Inc.
NDC code: 67226-2822
Medically reviewed by Drugs.com. Last updated on Nov 5, 2024.
2.0% Hydroquinone USP
Skin Bleaching and Correcting
- To gradually lighten hyperpigmentation of the skin such as age spots, liver spots, freckles or hyperpigmentation that can occur as a result of pregnancy or from the use of oral contraceptives.
For external use only.Use only on the advice of a physician.
- On broken or irritated skin.
- On children under 12 years of age.
- If you are pregnant or nursing unless instructed by a physician.
Contains Sodium Metabisulfite.
Limit sun exposure during treatment by using protective clothing or a sunscreen protective agent to avoid re-darkening of the skin.
- A mild transient stinging may occur for people with sensitive skin.
- Gradual lightening of the discolored area can be expected in most cases.
- Avoid contact with eyes and mucous membranes. In case of contact, rinse thoroughly with water.
- If irritation or rash occurs.
- Use fingertips to apply a thin layer to affected areas.
- Use both morning and night or as directed by a physician.
- Close cap securely after each use.
Butylated Hydroxy Toluene, Butylparaben, Cetearyl Alcohol, Cetyl Alcohol, Disodium EDTA, Ethoxydiglycol, Fragrance, Glycerin, Lactic Acid, L-Ascorbic Acid USP, Methylparaben, Phenyl Trimethicone, PPG-2 Myristyl Ether Propionate, Propylparaben, Sodium Cetearyl Sulfate, Sodium Lauryl Sulfate, Sodium Metabisulfite, Tocopheryl Acetate, Water/Eau.
450 455 9779 1 877 484 8437
Store at room temperature (15-30 oC / 59-86 oF). See package insert for complete details.

| CORRECTOR 2
hydroquinone cream |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Vivier Pharma Inc. (250996550) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Entreprises ImportFAB Inc. | 248586117 | manufacture(67226-2822) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
