Eye Stream
Dosage form: solution
Ingredients: Water 986mg in 1mL
Labeler: Alcon Laboratories, Inc.
NDC code: 0065-0530
Medically reviewed by Drugs.com. Last updated on Nov 12, 2024.
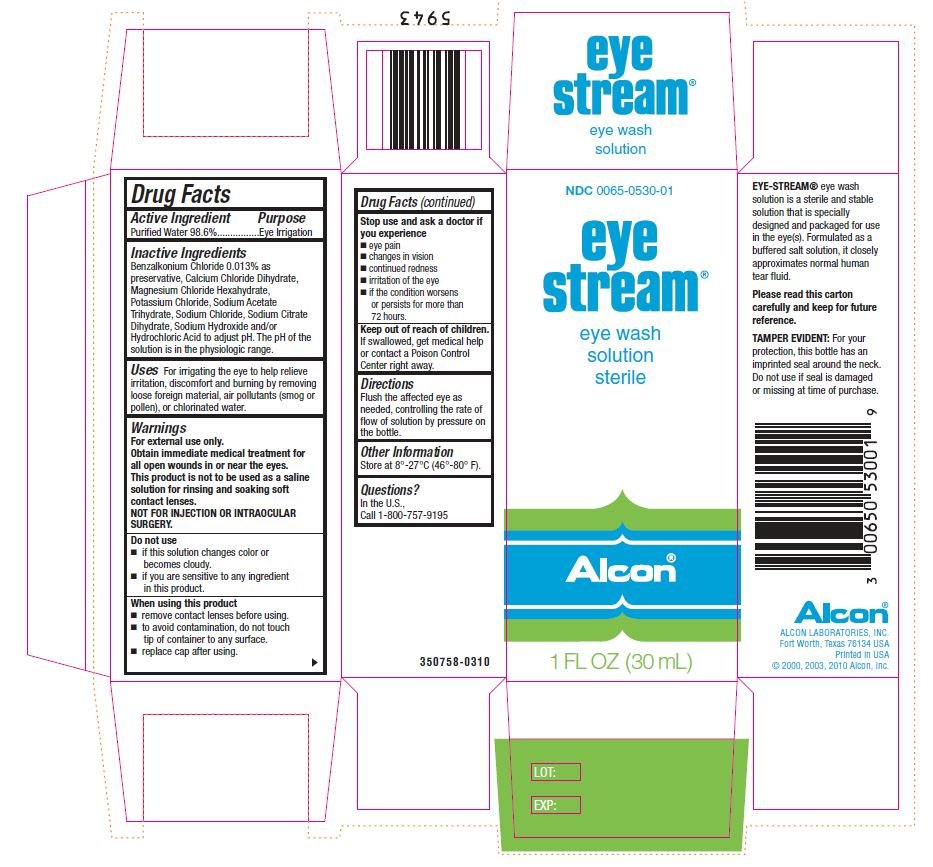
Drug Facts
NDC 0065-0530-01
| Active Ingredient | Purpose |
| Purified Water 98.6% | Eye Irrigation |
Benzalkonium Chloride 0.013% as preservative, Calcium Chloride Dihydrate, Magnesium Chloride Hexahydrate, Potassium Chloride, Sodium Acetate Trihydrate, Sodium Chloride, Sodium Citrate Dihydrate, Sodium Hydroxide and/or Hydrochloric Acid to adjust pH. The pH range of solution is in the physiologic range.
For irrigating the eye to help relieve irritation, discomfort and burning by removing loose foreign material, air pollutants (smog or pollen), or chlorinated water.
For external use only
Obtain immediate medical treatment for all open wounds in or near the eyes.
This product is not to be used as a saline solution for rinsing and soaking soft contact lenses.
NOT FOR INJECTION OR INTRAOCULAR SURGERY.
- if solution changes color or becomes cloudy.
- if you are sensitive to any ingredient in this product.
- remove contact lenses before using.
- to avoid contamination, do not touch tip of container to any surface.
- replace cap after using.
- eye pain
- changes in vision
- continued redness
- irritation of the eye
- if the condition worsens or persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Flush the affected eye as needed, controlling the rate of flow of solution by pressure on the bottle.
Store at 8o – 27oC (46o – 80oF)
In the U.S.,
Call 1-800-757-9195
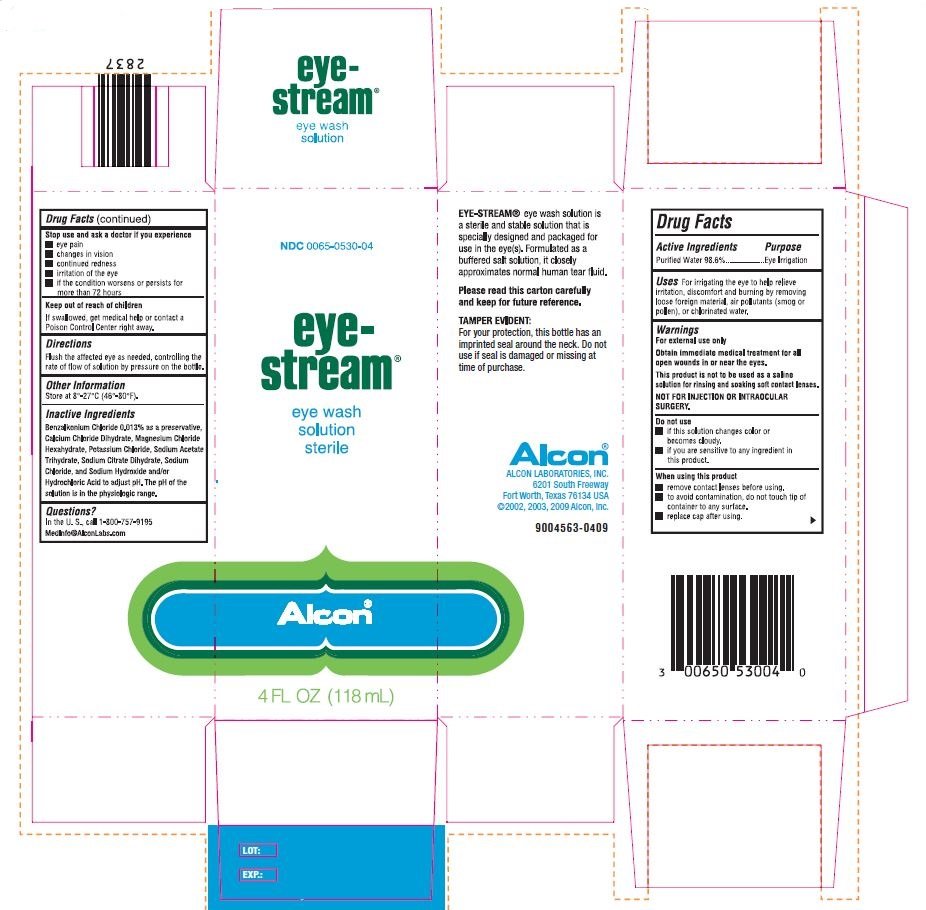
Drug Facts
NDC 0065-0530-04
| Active Ingredients | Purpose |
| Purified Water 98.6% | Eye Irrigation |
For irrigating the eye to help relieve irritation, discomfort and burning by removing loose foreign material, air pollutants, (smog or pollen), or chlorinated water.
For external use only
Obtain immediate medical treatment for all open wounds in or near the eyes.
This product is not to be used as a saline solution for rinsing and soaking soft contact lenses.
NOT FOR INJECTION OR INTRAOCULAR SURGERY.
- if this solution changes color or becomes cloudy.
- if you are sensitive to any ingredient in this product.
- remove contact lenses before using.
- to avoid contamination, do not touch tip of container to any surface.
- replace cap after using
- eye pain
- changes in vision
- continued redness
- irritation of the eye
- if the condition worsens or persists for more than 72 hours
If swallowed, get medical help or contact a Poison Control Center right away.
Flush the affected eye as needed, controlling the rate of flow of solution by pressure on the bottle.
Store at 8°-27°C (46°-80° F)
Benzalkonium Chloride 0.013% as a preservative, Calcium Chloride Dihydrate, Magnesium Chloride Hexahydrate, Potassium Chloride, Sodium Acetate Trihydrate, Sodium Citrate Dihydrate, Sodium Chloride and Sodium Hydroxide and/or Hydrochloric Acid to adjust pH. The pH of the solution is in the physiological range.
In the U.S., Call 1-800-757-9195
MedInfo@AlconLabs.com
NDC 0065-0530-01
eye stream®
eye wash solution sterile
Alcon®
1 FL OZ (30 mL)
NDC 0065-0530-04
eye stream®
eye wash solution
sterile
Alcon®
4 FL OZ (118 mL)
| EYE STREAM
purified water solution |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Alcon Laboratories, Inc. (008018525) |
| Registrant - Alcon Laboratories, Inc. (008018525) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Alcon Research LLC | 007672236 | manufacture(0065-0530) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
