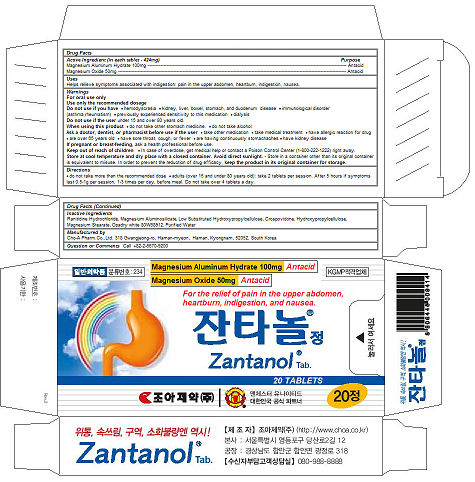
Zantanol Tab.
Dosage form: tablet
Ingredients: MAGNESIUM OXIDE 50mg, MAGALDRATE 100mg
Labeler: Cho-A Pharm.Co.,Ltd.
NDC code: 58354-120
Medically reviewed by Drugs.com. Last updated on Jan 20, 2025.
Magnesium Aluminum Hydrate 100mg
Magnesium Oxide 50mg
Antacid
• In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Helps relieve symptoms associated with indigestion: pain in the upper abdomen, heartburn, indigestion, nausea.
For oral use only
Use only the recommended dosage
Do not use if you have
• hemodyscrasia
• kidney, liver, bowel, stomach, and duodenum disease
• immunological disorder (asthma,rheumatism)
• previously experienced sensitivity to this medication
• dialysis
Do not use if the user under 15 and over 80 years old.
When using this product
• do not take other stomach medicine.
• do not take alcohol
Ask a doctor, dentist, or pharmacist before use if the user
• take other medication
• take medical treatment
• have allergic reaction for drug
• are over 65 years old
• have sore throat, cough, or fever
• are having continuously stomachaches
• have kidney disease
If pregnant or breast-feeding, ask a health professional before use.
Store at cool temperature and dry place with a closed container. Avoid direct sunlight. - Store in a container other than its original container is equivalent to misuse. In order to prevent the reduction of drug efficacy, keep the product in its original container for storage.
• do not take more than the recommended dose
• adults (over 15 and under 80 years old): take 2 tablets per session. After 5 hours if symptoms last 0.5-1g per session, 1-3 times per day, before meal. Do not take over 4 tablets a day.
Ranitidine Hydrochloride, Magnesium Aluminosilicate, Low Substituted Hydroxypropylcellulose, Crospovidone, Hydroxypropylcellulose, Magnesium Stearate, Opadry white 80W68912, Purified Water
| ZANTANOL TAB.
magnesium oxide, magnesium aluminum hydrate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Cho-A Pharm.Co.,Ltd. (688056831) |
| Registrant - Cho-A Pharm.Co.,Ltd. (688056831) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Cho-A Pharm.Co.,Ltd. | 688056831 | manufacture(58354-120) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
