Wet Nap Antibacterial Hand Wipes
Dosage form: cloth
Ingredients: BENZALKONIUM CHLORIDE 0.13mg in 1mL
Labeler: Professional Disposables International Inc.
NDC code: 10819-7012
Medically reviewed by Drugs.com. Last updated on Aug 14, 2025.
Benzalkonium chloride 0.13% w/w
Antiseptic handwash
For hand washing to decrease bacteria on the skin.
For external use only.
Do not use in the eyes.
Discontinue use if irritation and redness develop. If condition persists for more than 72 hours consult a doctor.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions for container:
- To dispense, lift cover, remove seal, pull center sheet from roll, twist to a point, feed through dispenser hole in cover. Keep lid closed to prevent moisture loss.
- Use as part of your hand cleaning routine.
- Run product onto hands and allow to dry.
- Discard after single use.
Directions for packets or flowrap: see on container the directions to retreive the wipe.
Purified Water, SD Alcohol 40, Sorbic Acid, PPG-2 Hydroxyethyl Cocamide, Disodium EDTA, Aloe Barbadensis Leaf Juice, Fragrance
Wet Naps
Antibacterial Hand Wipes
Moisturizes with Aloe
Kills 99.99% of Germs
Citrus Scent
Front Canister:

Back Canister:

Packet:
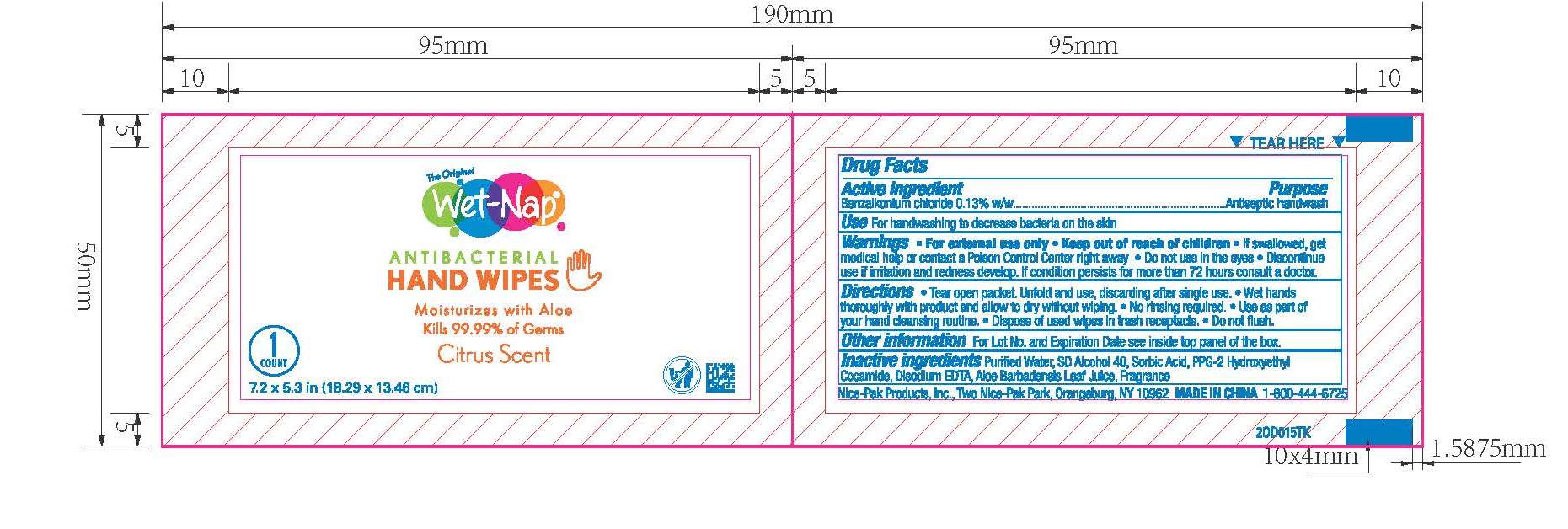
Box:

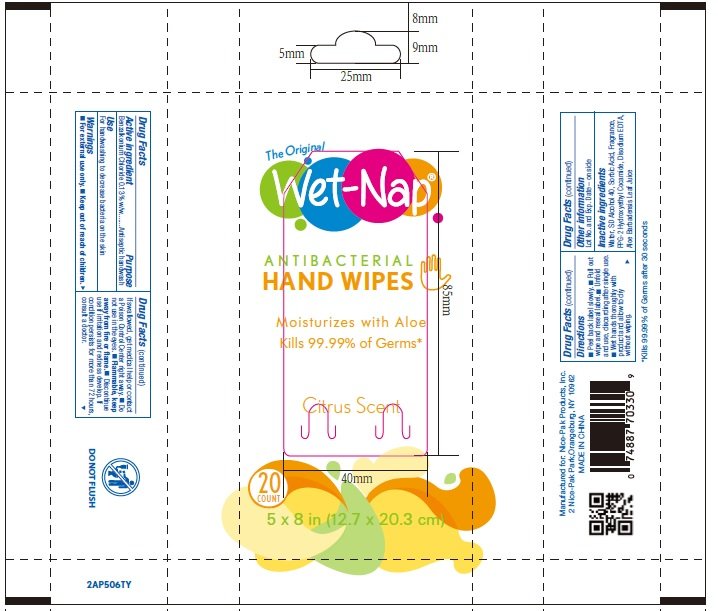
flowrap:
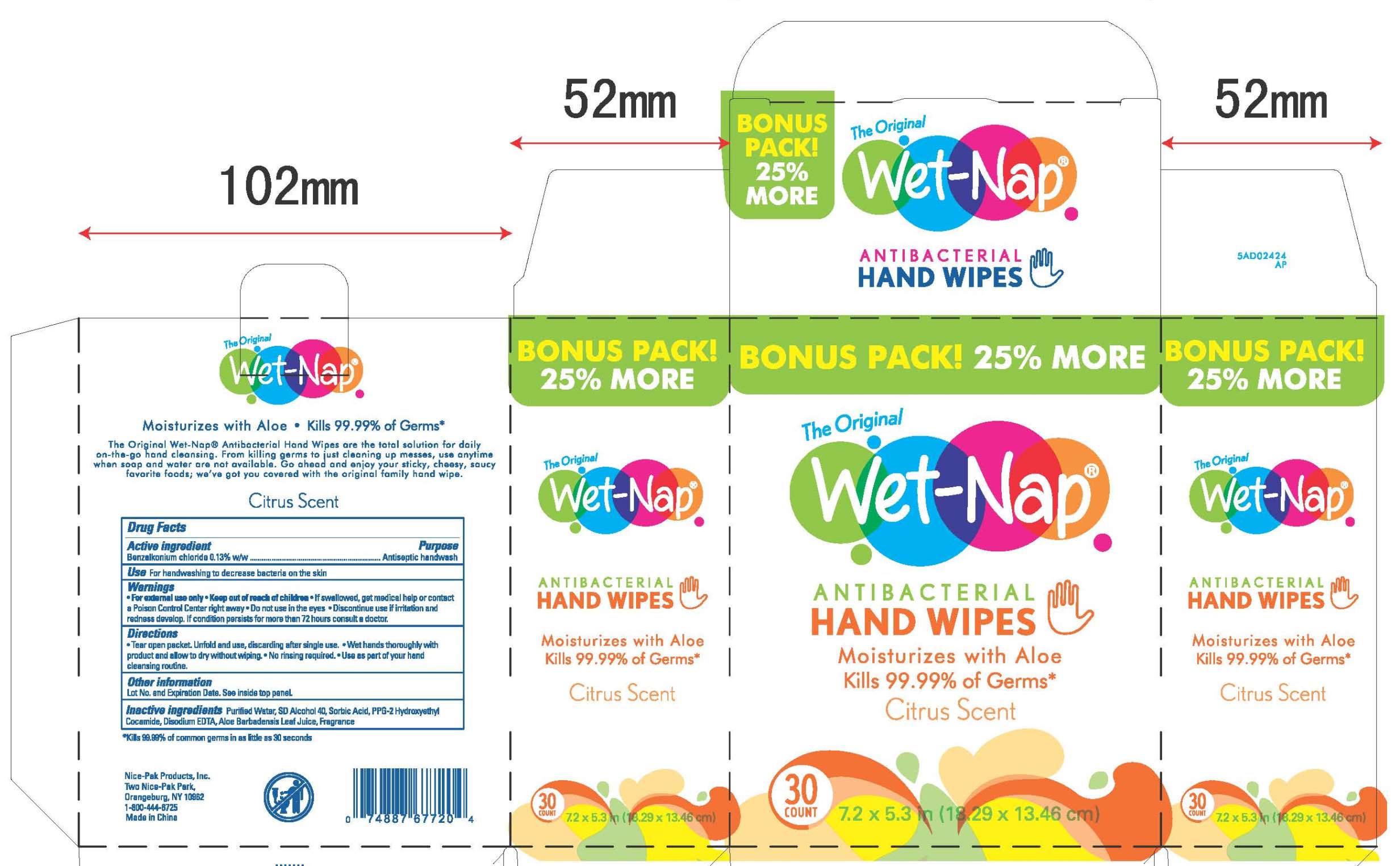
Bonus 30ct box:
Bonux 54ct canister:

| WET NAP
ANTIBACTERIAL HAND WIPES
benzalkonium chloride cloth |
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| Labeler - Professional Disposables International Inc. (800777117) |
| Registrant - Professional Disposables International Inc. (800777117) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
