CURODONT REPAIR FLUORIDE PLUS
Dosage form: sponge
Ingredients: SODIUM MONOFLUOROPHOSPHATE 0.05g in 100mL
Labeler: CREDENTIS AG
NDC code: 72247-105
Medically reviewed by Drugs.com. Last updated on Dec 27, 2024.
SODIUM FLUORIDE 0.05% (0.02% W/V FLUORIDE ION)
ANTICAVITY
AIDS IN THE PREVENTION OF DENTAL CAVITIES
- ANTICAVITY DENTAL RINSE
- RESTORES ENAMEL
- FOR THE TREATMENT OF WHITE SPOTS
FOR TOPICAL INTRAORAL USE ONLY. FOR PROFESSIONAL OFFICE USE ONLY. THIS PRODUCT IS NOT INTENDED FOR HOME OR UNSUPERVISED CONSUMER USE.
KEEP OUT OF REACH OF CHILDREN.
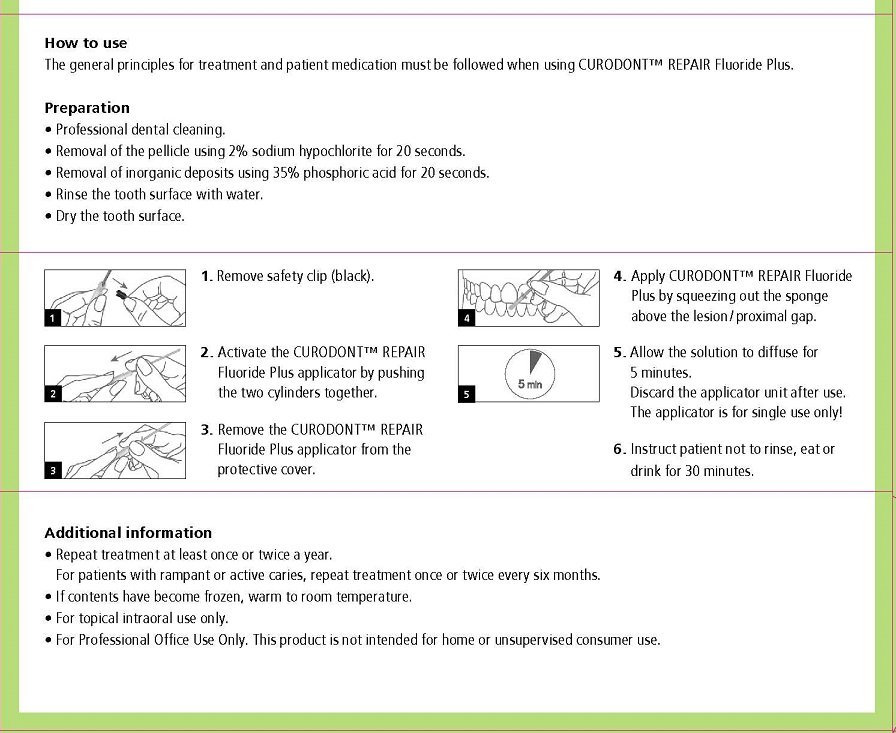
Usual dosage is one treatment per tooth. After prophylaxis treatment, remove the pellicle of the concerned tooth by using 2% sodium hypochlorite for 10 seconds. rinse the tooth surface with water. Remove inorganic deposits by pushing together the two cylinders and apply product with sponge on treatment area.
After treatment time of five minutes have patient expectorate residues. For maximum benefits for the prevention of caries, instruct patient not to rinse, eat or drink for 30 minutes. For patients with rampant or active caries, repeat treatment once or twice every six months.
DO NOT USE IF SAFETY PACHAGING IS BROKEN OR MISSING.
SINGLE USE ONLY.
STORE IN A DRY AND COOL PLACE.
WATER, CHLORHEXIDINE DIGLUCONATE, TROMETHAMINE, TREHALOSE DIHYDRATE, OLIGOPEPTIDE-104, HYDROXYPROPYL METHYLCELLULOSE


| CURODONT REPAIR FLUORIDE PLUS
sodium fluoride sponge |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - CREDENTIS AG (485450345) |
| Registrant - CREDENTIS AG (485450345) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| System Kosmetik Produktionsgesellschaft fur kosmetische Erzeugnisse mbH | 328825757 | manufacture(72247-105) | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| VALIDA | 480120305 | pack(72247-105) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
