HUMCO Isopropyl Rubbing Alcohol 70%
Dosage form: liquid
Ingredients: ISOPROPYL ALCOHOL 700mg in 1mL
Labeler: Humco Holding Group, Inc.
NDC code: 0395-1249
Medically reviewed by Drugs.com. Last updated on Mar 7, 2025.
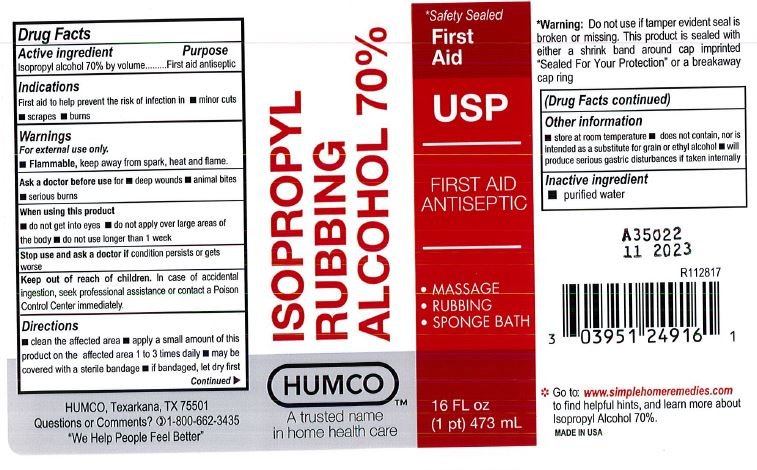
Drug Facts
Active Ingredient
Isopropyl Alcohol 70% by volume
Purpose
First aid antiseptic
Use
First aid to help prevent the risk of infection in.
- minor cuts
- scrapes
- burns
Warnings
For external use only.
- Flammable, keep way from spark, heat and flame.
Ask a doctor before use for
- deep wounds
- animal bites
- serious burns
When using this product
- do not get into eyes
- do not apply over large areas of the body
- do not use longer than 1 week
Stop use and ask a doctor if
condition persists or gets worse
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions
- clean the affected area.
- apply a smal amount of this product on the affected area 1 to 3 times daily.
- may be covered with sterile bandage.
- if bandaged, let it dry first.
Other Information
- store at room temperature
- does not contain, nor is intended as a substitute for grain or ethyl alcohol.
- will produce serious gastric distrurbances if taken internally.
Inactive Ingredient
purified water
Principal Display Panel
| HUMCO ISOPROPYL RUBBING ALCOHOL 70%
isopropyl alcohol liquid |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Humco Holding Group, Inc. (825672884) |
| Registrant - Humco Holding Group, Inc. (825672884) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Humco Holding Group, Inc. | 825672884 | manufacture(0395-1249), analysis(0395-1249), pack(0395-1249), label(0395-1249) | |
Document Id: a1116649-9101-3aa3-e053-2a95a90ae7fe
Set id: c220b916-6914-435f-84e2-ba828c9916b1
Version: 4
Humco Holding Group, Inc.
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
