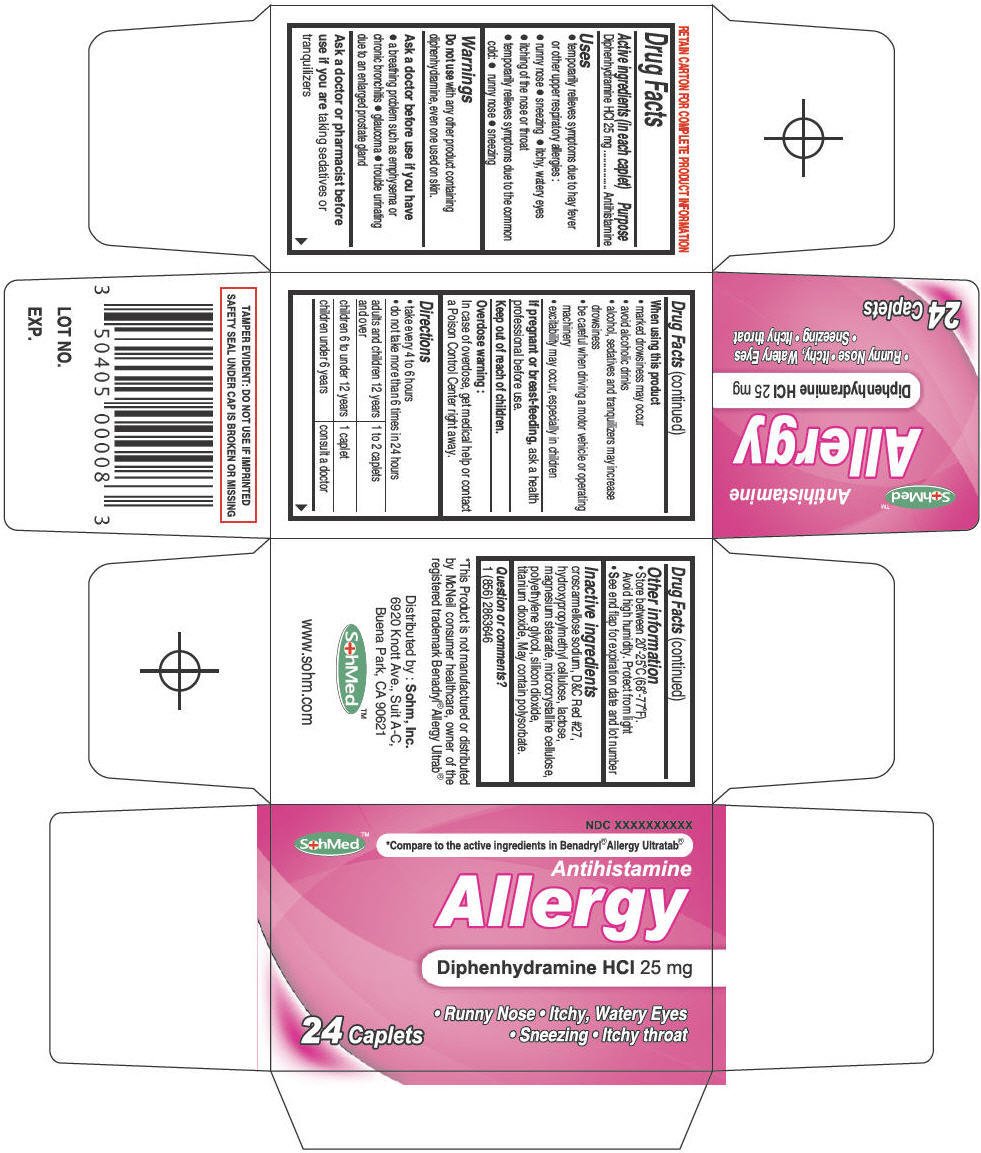
SohMed Allergy
Dosage form: tablet
Ingredients: Diphenhydramine Hydrochloride 25mg
Labeler: SOHM Inc.
NDC code: 50405-004
Medically reviewed by Drugs.com. Last updated on Aug 11, 2025.
Drug Facts
Diphenhydramine HCl 25 mg
Antihistamine
- temporarily relieves symptoms due to hay fever or other upper respiratory allergies :
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily relieves symptoms due to the common cold:
- runny nose
- sneezing
Do not use with any other product containing diphenhydramine, even one used on skin.
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
- take every 4 to 6 hours
- do not take more than 6 times in 24 hours
| adults and children 12 years and over | 1 to 2 caplets |
| children 6 to under 12 years | 1 caplet |
| children under 6 years | consult a doctor |
- Store between 20°-25°C (68°-77°F).
Avoid high humidity. Protect from light - See end flap for expiration date and lot number
croscarmellose sodium, D&C Red #27, hydroxypropylmethyl cellulose, lactose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, silicon dioxide, titanium dioxide, May contain polysorbate.
1 (856) 2863646
Distributed by : Sohm, Inc.
6920 Knott Ave., Suit A-C,
Buena Park, CA 90621
NDC XXXXXXXXXX
SohMed™
*Compare to the active ingredients in Benadryl® Allergy Ultratab®
Antihistamine
Allergy
Diphenhydramine HCl 25 mg
24 Caplets
• Runny Nose • Itchy, Watery Eyes
• Sneezing • Itchy throat
| SOHMED ALLERGY
diphenhydramine hydrochloride tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - SOHM Inc. (009303848) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
