Mary Kay TimeWise Day Solution SPF 30 Broad Spectrum
Dosage form: lotion
Ingredients: AVOBENZONE 2g in 100mL, HOMOSALATE 10g in 100mL, OCTISALATE 5g in 100mL, OCTOCRYLENE 2g in 100mL, OXYBENZONE 4g in 100mL
Labeler: Mary Kay Inc.
NDC code: 51531-5231
Medically reviewed by Drugs.com. Last updated on Aug 18, 2025.
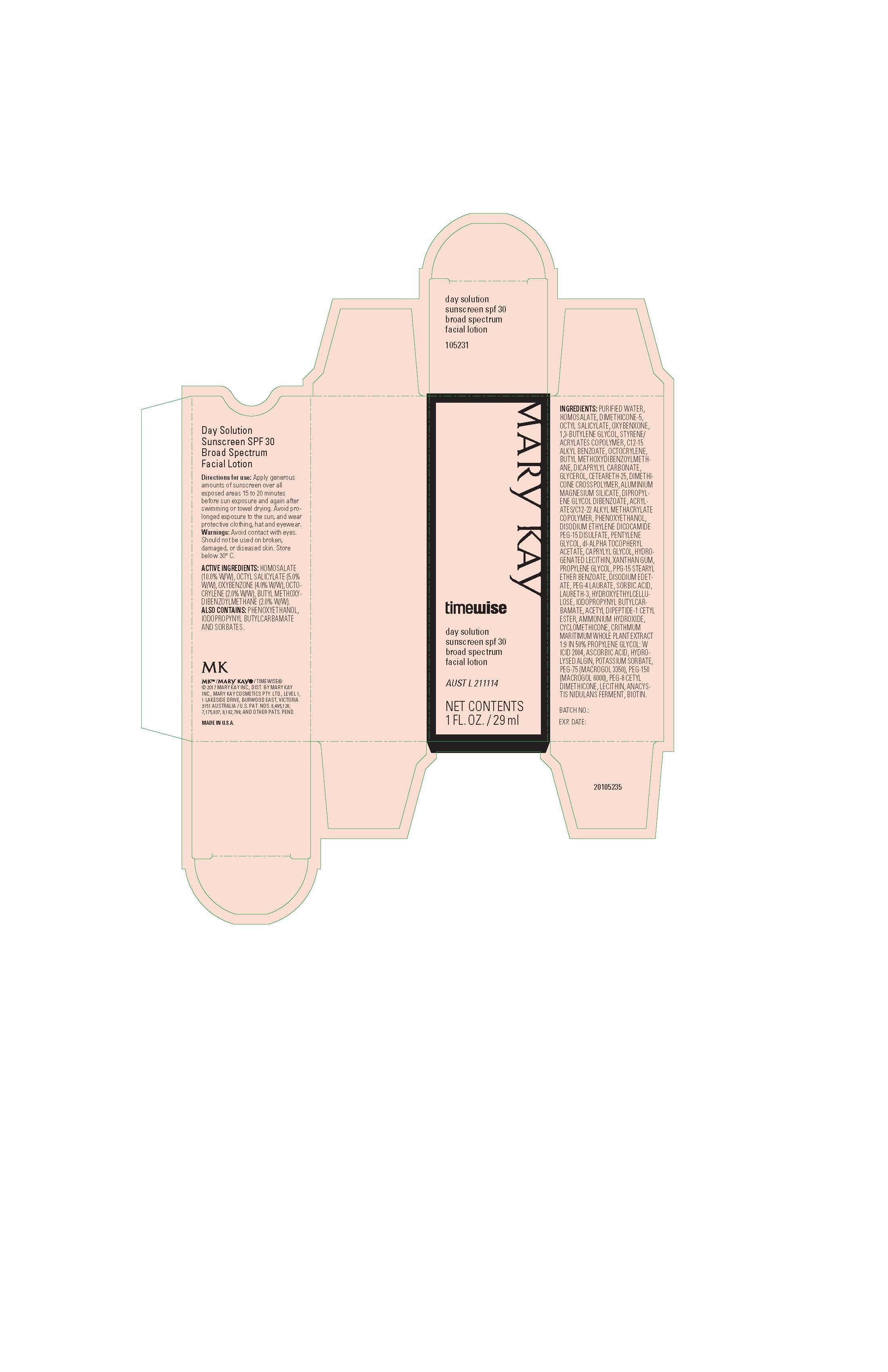
HOMOSALATE (10.0% W/W)
OCTYL SALICYLATE (5.0% W/W)
OXYBENZONE (4.0% W/W)
OCTOCRYLENE (2.0% W/W)
BUTYL METHOXYDIBENZOYLMETHANE (2.0% W/W)
Avoid contact with eyes. Should not be used on broken, damaged, or diseased skin. Store below 30° C.
Apply generous amounts of sunscreen over all exposed areas 15 to 20 minutes
before sun exposure and again after swimming or towel drying. Avoid prolonged exposure to the sun, and wear
protective clothing, hat and eyewear.
PURIFIED WATER, HOMOSALATE, DIMETHICONE-5, OCTYL SALICYLATE, OXYBENXONE, 1,3-BUTYLENE GLYCOL, STYRENE/ACRYLATES COPOLYMER, C12-15 ALKYL BENZOATE, OCTOCRYLENE, BUTYL METHOXYDIBENZOYLMETHANE, DICAPRYLYL CARBONATE, GLYCEROL, CETEARETH-25, DIMETHICONE CROSSPOLYMER, ALUMINIUM MAGNESIUM SILICATE, DIPROPYLENE GLYCOL DIBENZOATE, ACRYLATES/C12-22 ALKYL METHACRYLATE COPOLYMER, PHENOXYETHANOL, DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE, PENTYLENE GLYCOL, dl-ALPHA TOCOPHERYL ACETATE, CAPRYLYL GLYCOL, HYDROGENATED LECITHIN, XANTHAN GUM, PROPYLENE GLYCOL, PPG-15 STEARYL ETHER BENZOATE, DISODIUM EDETATE, PEG-4 LAURATE, SORBIC ACID, LAURETH-3, HYDROXYETHYLCELLULOSE, IODOPROPYNYL BUTYLCARBAMATE, ACETYL DIPEPTIDE-1 CETYL ESTER, AMMONIUM HYDROXIDE, CYCLOMETHICONE, CRITHMUM MARITIMUM WHOLE PLANT EXTRACT 1:9 IN 50% PROPYLENE GLYCOL: W ICID 2004, ASCORBIC ACID, HYDROLYSED ALGIN, POTASSIUM SORBATE, PEG-75 (MACROGOL 3350), PEG-150 (MACROGOL 6000), PEG-8 CETYL DIMETHICONE, LECITHIN, ANACYSTIS NIDULANS FERMENT, BIOTIN.
Mary Kay
timewise
day solution
sunscreen spf 30
broad spectrum
facial lotion
AUST L 211114
NET CONTENTS
1 FL. OZ. / 29 ml
| MARY KAY TIMEWISE DAY SOLUTION SPF 30
BROAD SPECTRUM
avobenzone, homosalate, octisalate, octocrylene, oxybenzone lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Mary Kay Inc. (049994452) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Englewood Labs Inc. | 080987545 | manufacture(51531-5231) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
