DR. RECKEWEG R81 Maldol Combination product
Dosage form: liquid
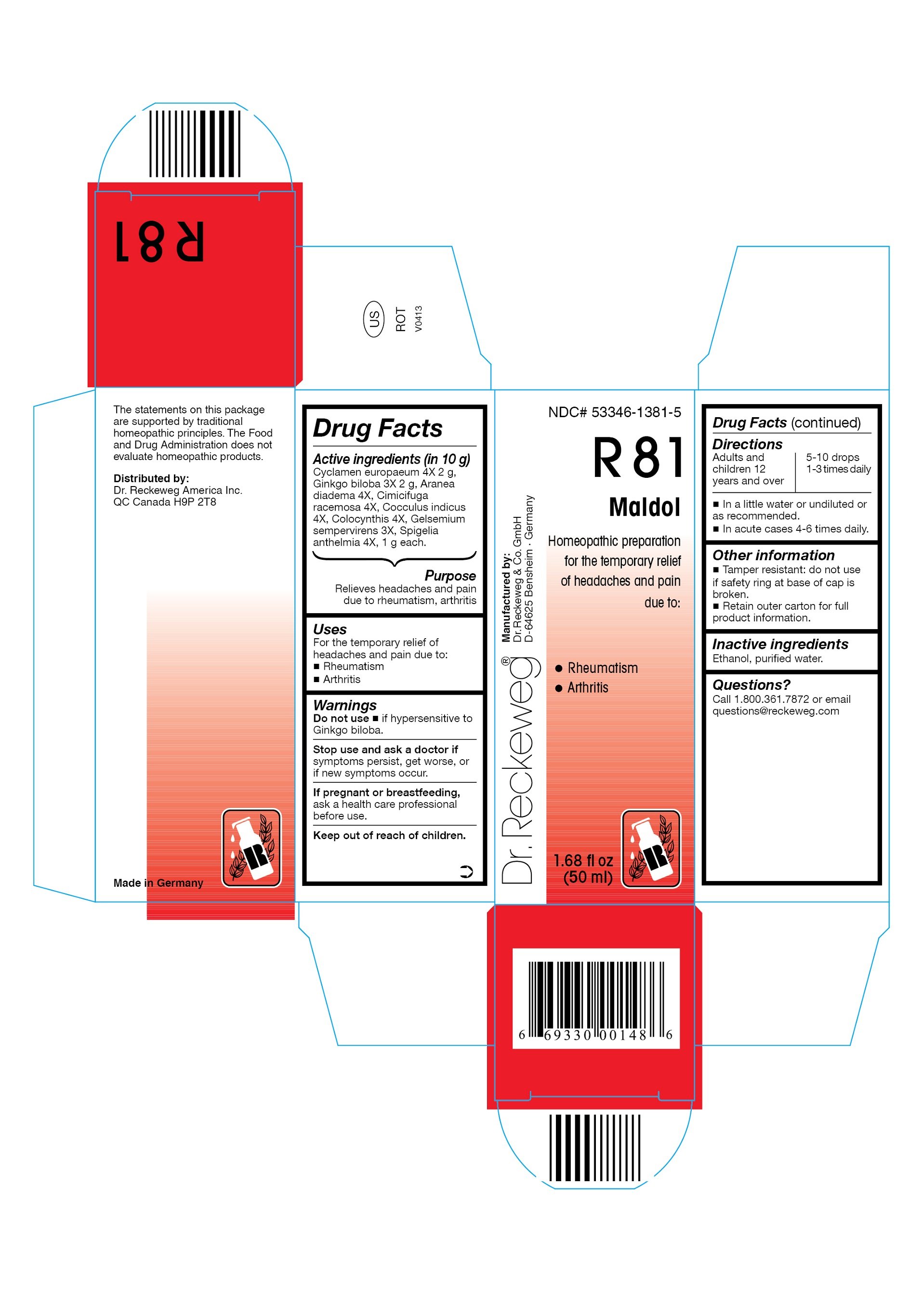
Ingredients: CYCLAMEN PURPURASCENS TUBER 4[hp_X] in 50mL, GINKGO 3[hp_X] in 50mL, ARANEUS DIADEMATUS 4[hp_X] in 50mL, BLACK COHOSH 4[hp_X] in 50mL, ANAMIRTA COCCULUS SEED 4[hp_X] in 50mL, CITRULLUS COLOCYNTHIS FRUIT PULP 4[hp_X] in 50mL, GELSEMIUM SEMPERVIRENS ROOT 3[hp_X] in 50mL, SPIGELIA ANTHELMIA 4[hp_X] in 50mL
Labeler: PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO
NDC code: 53346-1381
Active ingredients
Cyclamen europaeum 4X 2 g, Ginkgo biloba 3X 2 g, Aranea diadema 4X, Cimicifuga racemosa 4X, Cocculus indicus 4X, Colocynthis 4X, Gelsemium sempervirens 3X, Spigelia anthelmia 4X, 1 g each in 10 g.
Purpose
Relieves headaches and pain due to rheumatism, arthritis
Uses
For the temporary relief of headaches and pain due to:
- Rheumatism
- Arthritis
Warnings
Do not use
- if hypersensitive to Ginkgo biloba.
Stop use and ask a doctor if symptoms persist, get worse, or if new symptoms occur.
If pregnant or breastfeeding, ask a health care professional before use.
Keep out of reach of children.
Directions
Adults and children ≥ 12 years 5-10 drops 1-3 times daily, in acute cases 4-6
times daily in a little water or undiluted or as recommended.
Other information
- Tamper resistant: do not use if safety ring at base of cap is broken.
- Retain outer carton for full product information.
Inactive ingredients
Ethanol, purified water.
Questions?
Call 1-800-361-7872 or email questions@reckeweg.com
| DR. RECKEWEG R81 MALDOL
COMBINATION PRODUCT
cyclamen europaeum 4x, ginkgo biloba 3x, aranea diadema 4x, cimicifuga racemosa 4x, cocculus indicus 4x, colocynthis 4x, gelsemium sempervirens 3x, spigelia anthelmia 4x liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO (318602612) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO | 318602612 | manufacture(53346-1381) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
See also:
Qulipta
Qulipta is used to help prevent episodic or chronic migraine headaches in adults. Qulipta is an ...
Aimovig
Learn about Aimovig (erenumab-aooe) a once-monthly, injectable medication that can be ...
Dupixent
Dupixent is used to treat eczema, eosinophilic or oral-corticosteroid-dependent asthma, chronic ...
Gemtesa
Gemtesa (vibegron) is used to treat overactive bladder symptoms (OAB) in adults or men taking ...
Ubrelvy
Ubrelvy (ubrogepant) tablets are used for the acute treatment of migraine. Includes Ubrelvy side ...
Nurtec ODT
Nurtec ODT (rimegepant) is used to treat acute migraines and prevent episodic migraines, by ...
Xeomin
Xeomin (incobotulinumtoxinA) is used to treat cervical dystonia, blepharospasm, upper facial lines ...
Tazarotene topical
Tazarotene topical is a type of retinoid derived from vitamin A that is available as cream, gel ...
Topiramate
Learn about topiramate, an anticonvulsant used for seizures, migraine prevention, and weight loss ...