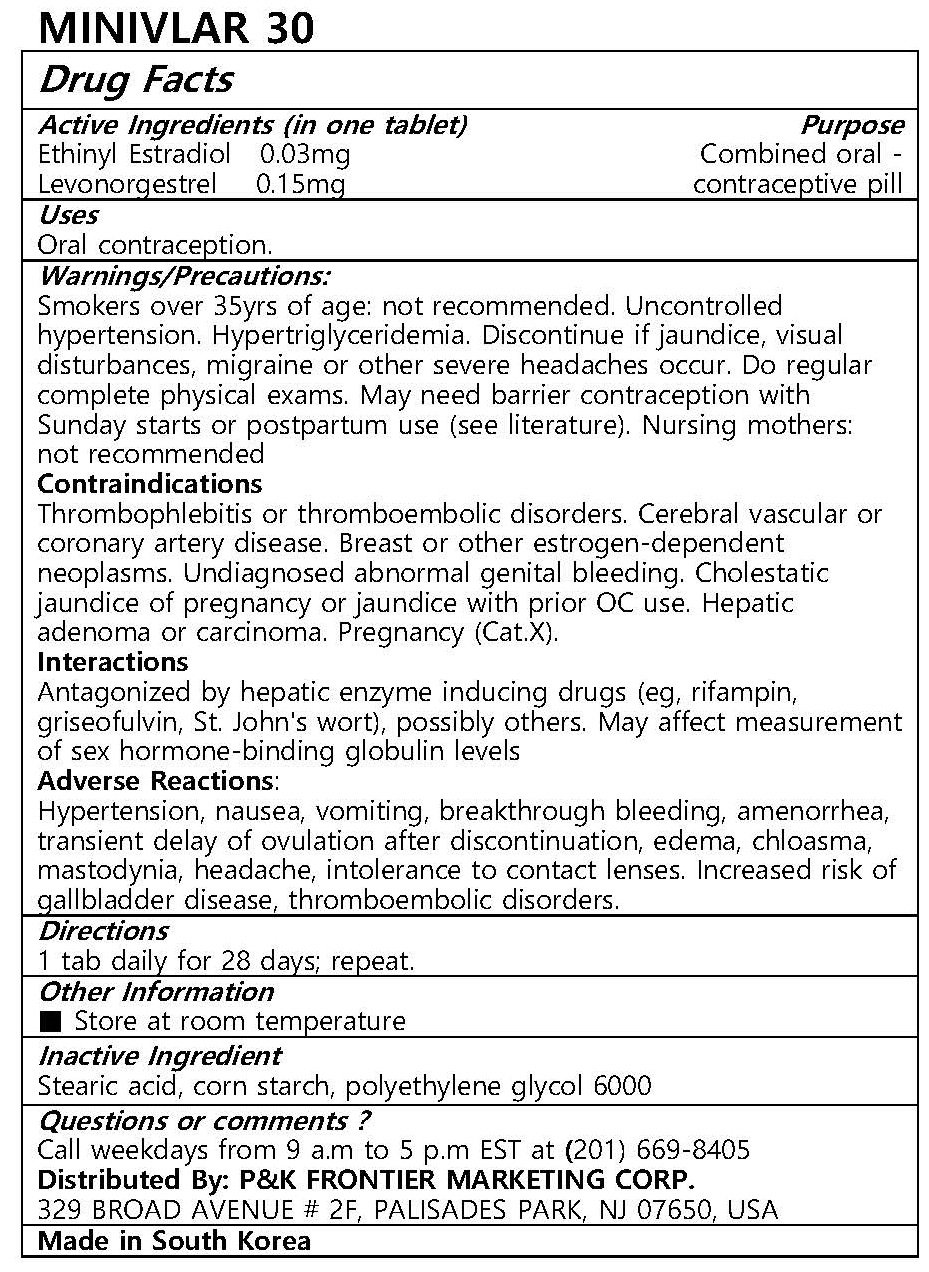
MINIVLAR 30
Dosage form: tablet
Ingredients: ETHINYL ESTRADIOL 0.03mg, LEVONORGESTREL 0.15mg
Labeler: OASIS TRADING
NDC code: 72689-0011
Medically reviewed by Drugs.com. Last updated on Nov 11, 2024.
Ethinyl Estradiol, Levonorgestrel
Oral contraception
Keep out of reach of children
1 tab daily for 21 days; repeat.
Warnings/Precautions:
Smokers over 35yrs of age: not recommended. Uncontrolled hypertension. Hypertriglyceridemia. Discontinue if jaundice, visual disturbances, migraine or other severe headaches occur. Do regular complete physical exams. May need barrier contraception with Sunday starts or postpartum use (see literature). Nursing mothers: not recommended
Contraindications
Thrombophlebitis or thromboembolic disorders. Cerebral vascular or coronary artery disease. Breast or other estrogen-dependent neoplasms. Undiagnosed abnormal genital bleeding. Cholestatic jaundice of pregnancy or jaundice with prior OC use. Hepatic adenoma or carcinoma. Pregnancy (Cat.X).
Interactions
Antagonized by hepatic enzyme inducing drugs (eg, rifampin, griseofulvin, St. John's wort), possibly others. May affect measurement of sex hormone-binding globulin levels
Adverse Reactions:
Hypertension, nausea, vomiting, breakthrough bleeding, amenorrhea, transient delay of ovulation after discontinuation, edema, chloasma, mastodynia, headache, intolerance to contact lenses. Increased risk of gallbladder disease, thromboembolic disorders.
Stearic acid, corn starch, polyethylene glycol 6000
For oral use only
| MINIVLAR 30
ethinyl estradiol, levonorgestrel tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - OASIS TRADING (689991468) |
| Registrant - OASIS TRADING (689991468) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| OASIS TRADING | 689991468 | manufacture(72689-0011), label(72689-0011) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
