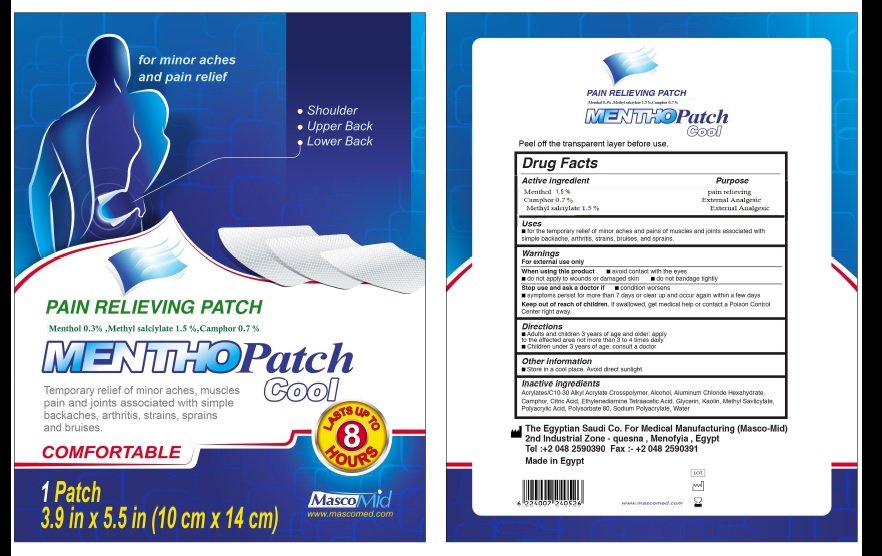
Mentho patch cool
Dosage form: patch
Ingredients: Menthol 15mg in 1g
Labeler: THE EGYPTIAN SAUDI CO FOR MEDICAL MANUFACTURING MASCOMID
NDC code: 72602-002
Medically reviewed by Drugs.com. Last updated on Oct 28, 2024.
For the temprorary releif of minor aches and pains of musceles and joints associated with simple backache, arthritis,strainsand sprains
- FOR EXTERNAL USE ONLY
- When Using this Product
- Avoid contact with eyes
- Do not apply to open wounds, or to damaged skin
- Do not bandage tightly.
- Adults and children 3 years of age and older: apply to affected area not more than 3 to 4 times daily.
- Children under 3 years of age: consult doctor.
- If condition worsens
- If symptoms persist more than 7 days or clear up and occur again with a few days
- store in cool dry place avoid direct sunlight.
If swallowed, get medical help or contact a Poison Control Center right away
Menthol,Camphor,Methyl salicylate
Glycerin ,Sodium poly acryl ate ,Aluminum chloride hex hydrate 2 ,
Anhydrous citric acid ,Tartaric acid 1 ,Poly sorbate -80 (tween 80) 0.5 ,Isopropyl myristate alcohol 2.5 ,Car boxy methyl cellulose (CMC) 2
EDETA 0.5 ,Polyvinyl pyrolidone(PVP-K90) 3 ,Water 48.35 ,titanium dioxide 0.05,ACRYLATE/ C10-30 ALKYL ACRYLATE,cross polymer
| MENTHO PATCH
COOL
menthol patch |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - THE EGYPTIAN SAUDI CO FOR MEDICAL MANUFACTURING MASCOMID (850486280) |
| Registrant - THE EGYPTIAN SAUDI CO FOR MEDICAL MANUFACTURING MASCOMID (850486280) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| THE EGYPTIAN SAUDI CO FOR MEDICAL MANUFACTURING MASCOMID | 850486280 | manufacture(72602-002) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
