BD PERSIST
Dosage form: solution
Ingredients: povidone-iodine 85mg in 1mL, alcohol 0.7mL in 1mL
Labeler: Becton, Dickinson and Company
NDC code: 17271-507
Medically reviewed by Drugs.com. Last updated on Sep 3, 2025.
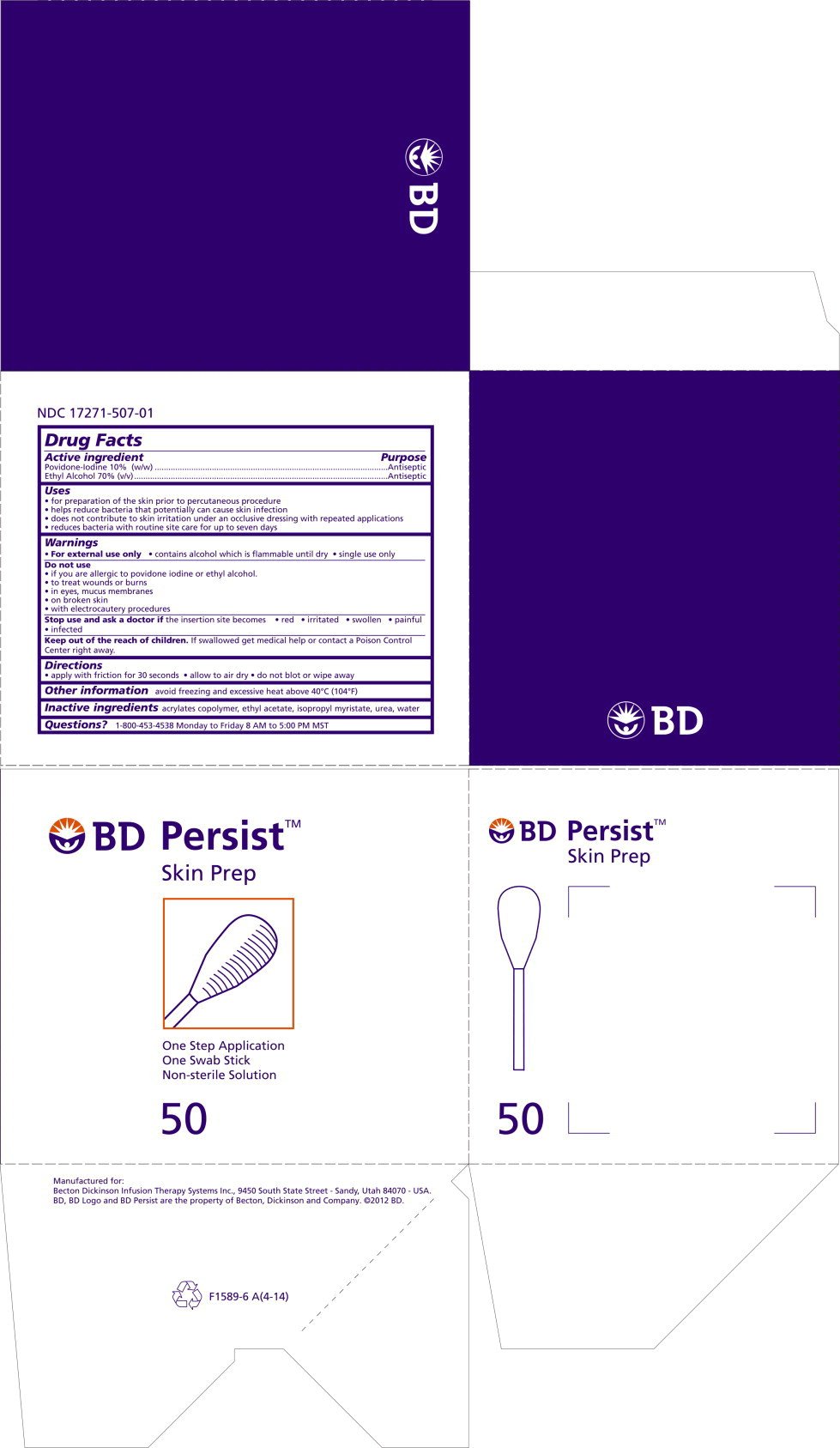
Drug Facts
Providone-Iodine 10% (w/w)
Ethyl Alcohol 70% (v/v)
Antiseptic
Antiseptic
- for preparation of the skin prior to percutaneous procedure
- helps reduce bacteria that potentially can cause skin infection
- does not contribute to skin irritation under an occlusive dressing with repeated applications
- reduces bacteria with routine site care for up to seven days
- For external use only
- contains alcohol which is flammable until dry
- Single use only
- if you are allergic to povidone iodine or ethyl alcohol
- to treat wounds or burns
- in eyes, mucus membranes
- on broken skin
- with electrocautery procedures
Stop use and ask a doctor if the insertion site becomes
- red
- irritated
- swollen
- painful
- infected
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- apply with friction for 30 seconds
- allow to dry
- do not blot or wipe away
acrylates copolymer, ethyl alcohol, isopropyl myristate, urea, water
Principal Display Panel – Carton Label
BD Persist™
Skin Prep
One Step Application
One Swab Stick
Non-sterile solution
50
Principal Display Panel – Package Label
BD Persist™ Skin Prep
1 Swab Stick • Non-sterile solution REF 386401 NDC 17271-507-01

| BD PERSIST
povidone-iodine, alcohol solution |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
| Labeler - Becton, Dickinson and Company (124987988) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Becton, Dickinson and Company | 124987988 | MANUFACTURE(17271-507) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
