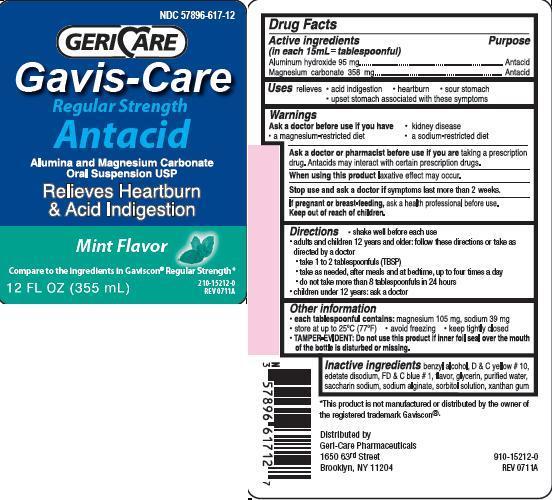
GAVIS-CARE ANTACID
Dosage form: suspension
Ingredients: ALUMINUM HYDROXIDE 95mg in 15mL, MAGNESIUM CARBONATE 358mg in 15mL
Labeler: Geri-Care Pharmaceuticals, Corp
NDC code: 57896-617
Medically reviewed by Drugs.com. Last updated on Dec 17, 2024.
Aluminum hydroxide 95 mg
Magnesium carbonate 358 mg
Antacid
relieves
- heartburn
- sour stomach
- acid indigestion
- upset stomach associated with these symptoms
Ask a doctor before use if you have
• kidney disease
• a magnesium-restricted diet
• a sodium-restricted diet
Ask a doctor or pharmacist before use if you are taking a prescription drug.
Antacids may interact with certain prescription drugs.
When using this product laxative effect may occur.
Stop use and ask a doctor if symptoms last more than 2 weeks
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
• shake well before each use
• adults and children 12 years and older: follow these directions or take as directed by a doctor
• take 1 to 2 tablespoonfuls (TBSP)
• take as needed, after meals and at bedtime, up to four times a day
• do not take more than 8 tablespoonfuls in 24 hours
• children under 12 years: ask a doctor
•
each tablespoonful contains: magnesium 105 mg, sodium 39 mg
• store at up to 25C (77F)
• avoid freezing
• keep tightly closed
•
TAMPER-EVIDENT: Do not use this product if inner foil seal over the mouth of the bottle is disturbed or missing.
benzyl alcohol, D and C yellow no.10, edetate disodium, FD and C blue no.1, flavor, glycerin, purified water, saccharin sodium, sodium alginate, sorbitol solution, xanthan gum
NDC 57896-617-12
GERICARE
Gavis-Care
Regular Strength
Antacid
Alumina and Magnesium Carbonate Oral Suspension USP
Relives Heartburn and Acid Indigestion
mint flavor
compare to the ingredients in Gaviscon Regular Strength
12 FL OZ (355 mL)
| GAVIS-CARE ANTACID
aluminum hydroxide and magnesium carbonate suspension |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Geri-Care Pharmaceuticals, Corp (611196254) |
| Registrant - GCP Laboratories (965480861) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| GCP Laboratories | 965480861 | manufacture(57896-617) | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
