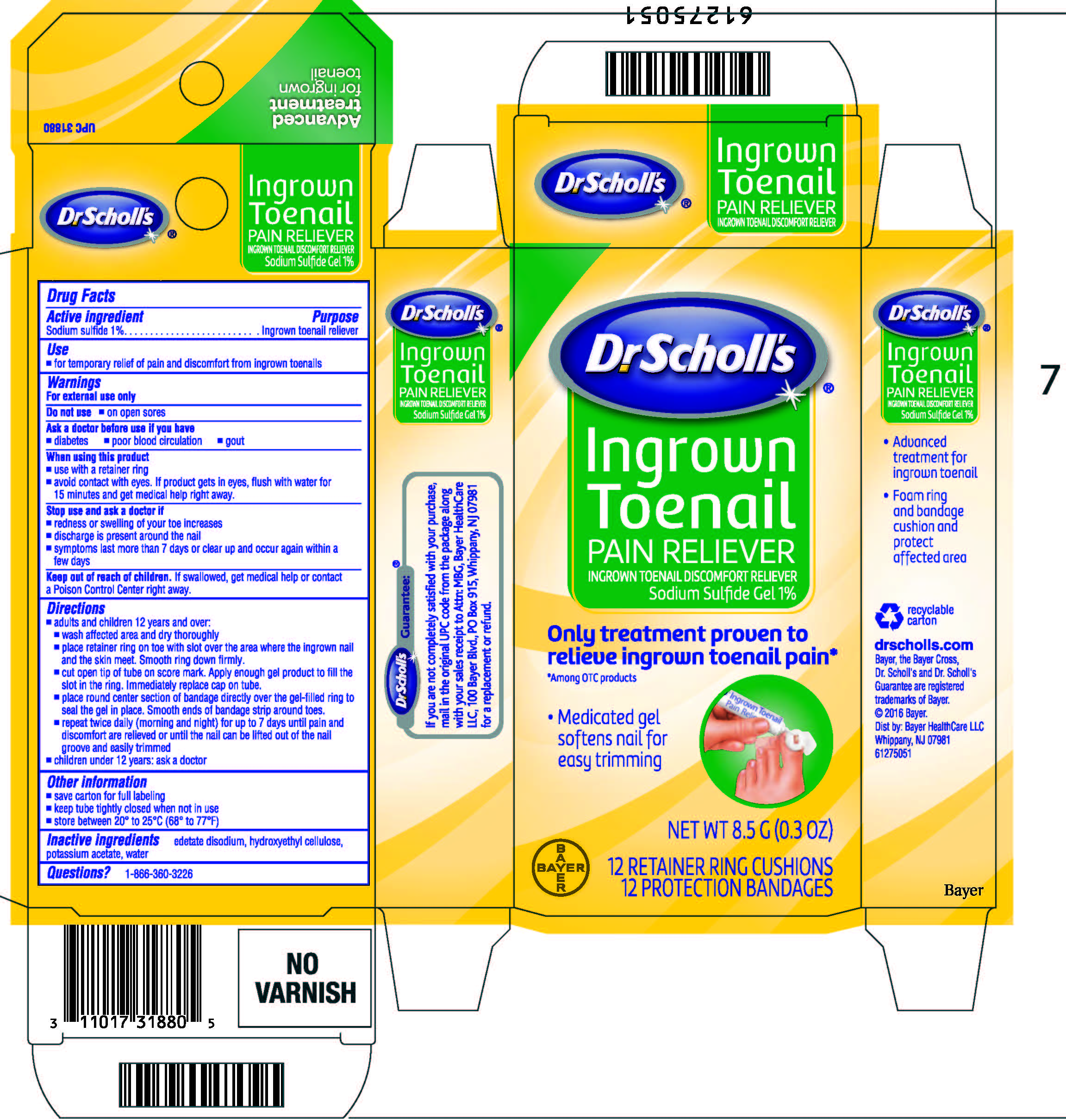
Dr. Scholls Ingrown Toenail Pain Reliever
Dosage form: gel
Ingredients: SODIUM SULFIDE 10mg in 1g
Labeler: Bayer HealthCare LLC.
NDC code: 11523-7214
Medically reviewed by Drugs.com. Last updated on Nov 25, 2024.
Ingrown Toenail Pain Reliever
Drug Facts
Sodium sulfide 1%
Ingrown toenail reliever
- for temporary relief of pain and discomfort from ingrown toenails
For external use only
Do not use on open sores
- diabetes
- poor blood circulation
- gout
- use with a retainer ring
- avoid contact with eyes. If product gets in eyes, flush with water for 15 minutes and get medical help right away.
- redness or swelling of your toe increases
- discharge is present around the nail
- symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- adults and children 12 years and over:
- wash affected area and dry thoroughly
- place retainer ring on toe with slot over the area where the ingrown nail and the skin meet. Smooth ring down firmly.
- cut open tip of tube on score mark. Apply enough gel product to fill the slot in the ring. Immediately replace cap on tube.
- place round center section of bandage directly over the gel-filled ring to seal the gel in place. Smooth ends of bandage strip around toe.
- repeat twice daily (morning and night) for up to 7 days until pain and discomfort is relieved or until the nail can be lifted out of the nail groove and easily trimmed
- children under 12 years: ask a doctor
- save carton for full labeling
- keep tube tightly closed when not in use
- store between 20° to 25°C (68° to 77°F)
edetate disodium, hydroxyethyl cellulose, potassium acetate, purified water
Distributed by Bayer HealthCare LLC
Whippany, NJ 07981
Dr. Scholl's®
Ingrown
Toenail
PAIN RELIEVER
Sodium Sulfide Gel 1%
Only Treatment Proven to
Relieve Ingrown Toenail Pain*
-
Medicated gel
softens nail for
easy trimming
NET WT 8.5 g (0.3 OZ)
12 RETAINER RING CUSHIONS
12 PROTECTION BANDAGES
| DR. SCHOLLS
INGROWN TOENAIL PAIN RELIEVER
sodium sulfide gel |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - Bayer HealthCare LLC. (112117283) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
