OMNI
Dosage form: gel
Ingredients: stannous fluoride 0.969mg in 1g
Labeler: 3M ESPE Dental Products
NDC code: 48878-4061
Medically reviewed by Drugs.com. Last updated on Jun 2, 2025.
Drug Facts
Active ingredient
Stannous fluoride 0.4% w/w (0.12% w/v fluoride ion)
Purpose
Anticavity
Use
Aids in the prevention of dental decay
Warnings
- This is a fluoride preventive treatment gel, not a toothpaste. Read directions carefully before using.
- Keep out of reach of children. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
- This product may produce surface staining of the teeth. Adequate tooth brushing may prevent these stains which are not harmful or permanent and may be removed by your dentist.
Directions
- Adults and children 6 years of age and older: Use a pea-sized (.25g) dose once a day after brushing your teeth with a toothpaste. Apply the gel to your teeth and brush thoroughly. Allow the gel to remain on your teeth for 1 minute and then spit out. Do not swallow the gel. Do not eat or drink for 30 minutes after brushing. Instruct children under 12 years of age in the use of this product (to minimize swallowing). Supervise children as necessary until capable of using without supervision.
- Children under 6 years of age: Consult a dentist or doctor.
Other information
- Do not freeze or expose to extreme heat.
- Do not use if tamper evident seal on top of box is broken or removed.
Inactive ingredients
ascorbic acid, carbomer, citric acid, flavor, glycerin, triethanolamine
Questions or comments?
call toll free M-F 9am to 5pm EST/EDT at 1-800-634-2249
Principle Display Panel
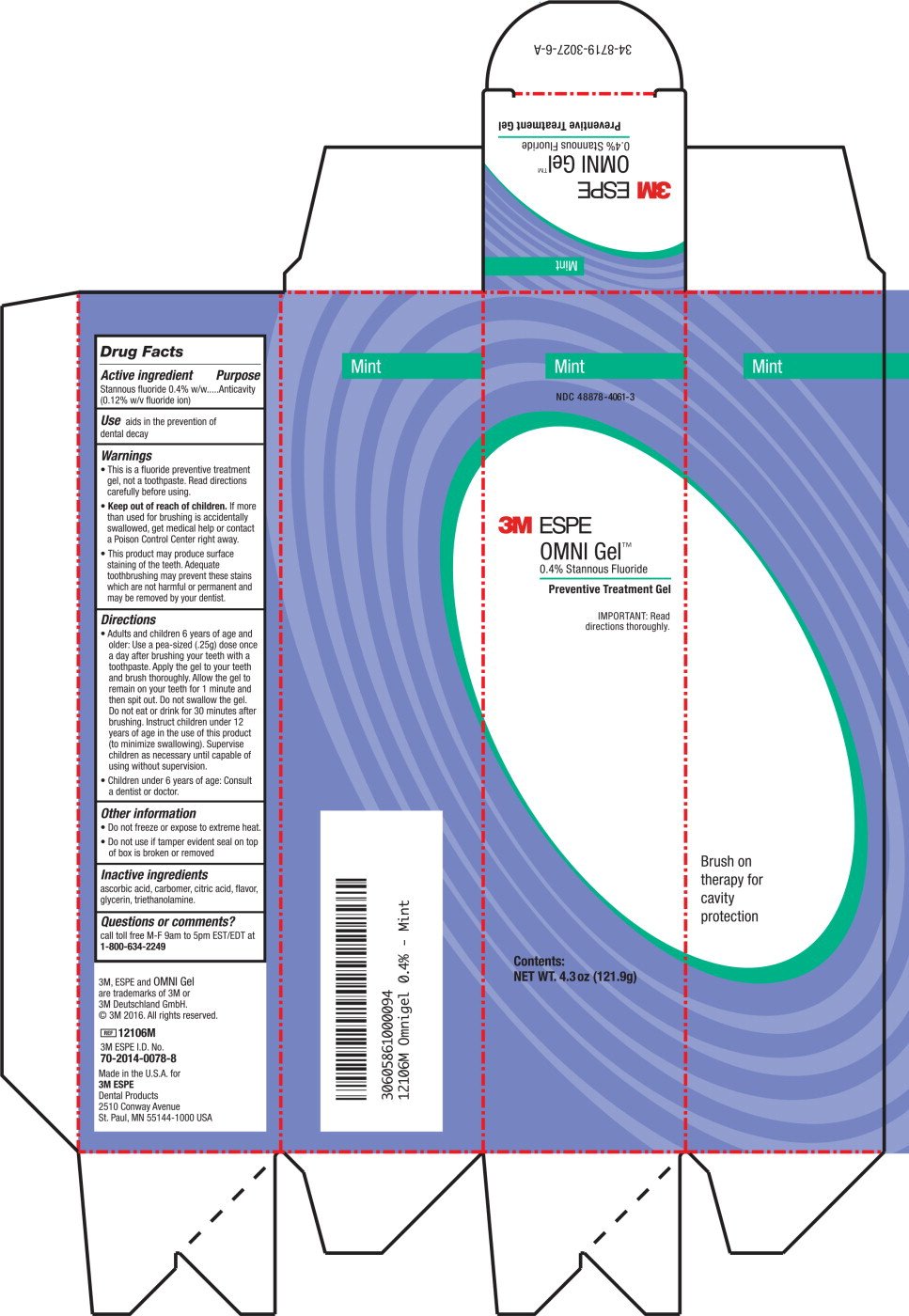
Mint
NDC 48878-4061-3
3M ESPE
OMNI Gel™
0.4% Stannous Fluoride
Preventive Treatment Gel
IMPORTANT: Read
Directions thoroughly.
Contents:
NET WT. 4.3 oz (121.9 g)

| OMNI
stannous fluoride gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - 3M ESPE Dental Products (801390852) |
Document Id: 5539e66a-f111-4d1d-b4fd-6d0eaab84a84
Set id: 0c2a81b1-65b5-432b-8e66-30432a245aba
Version: 5
3M ESPE Dental Products
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
