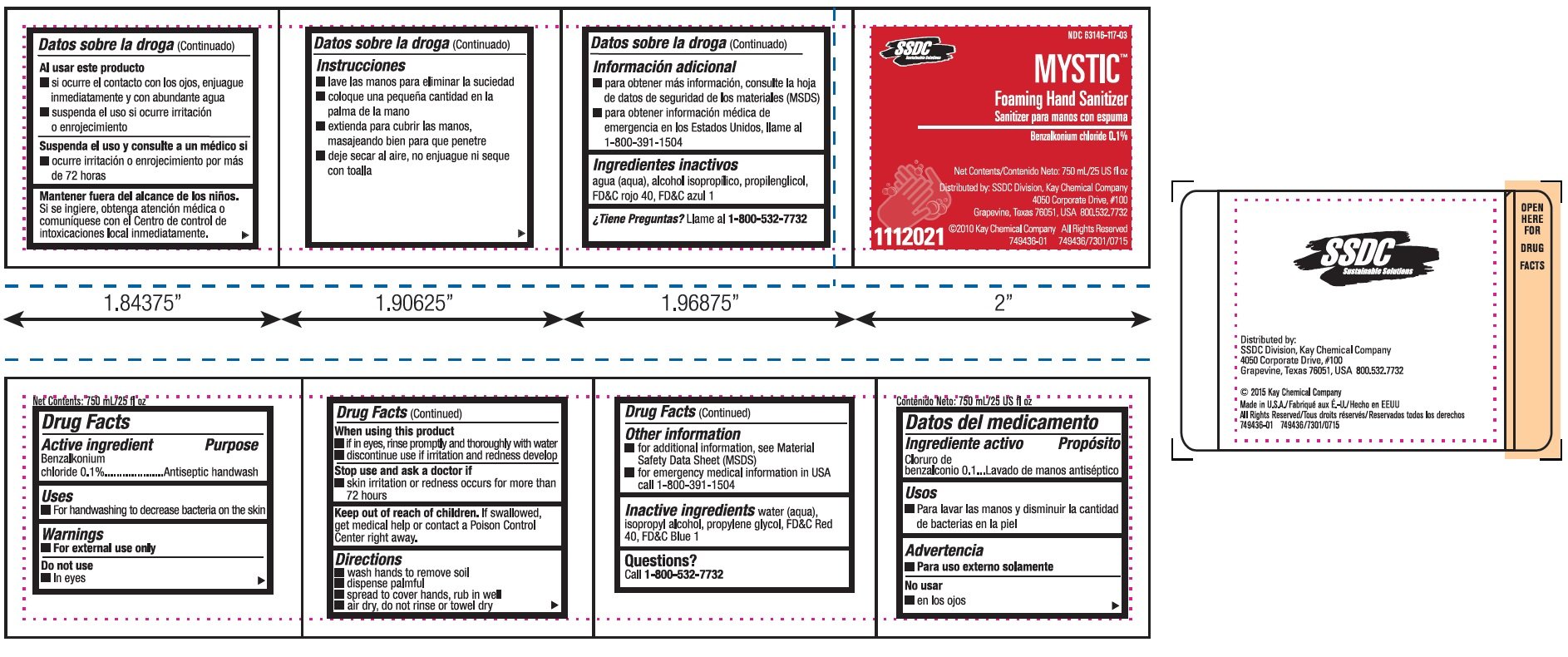
Mystic Foaming Hand Sanitizer
Dosage form: solution
Ingredients: BENZALKONIUM CHLORIDE 1mg in 1mL
Labeler: Kay Chemical Company
NDC code: 63146-117
Medically reviewed by Drugs.com. Last updated on Aug 1, 2025.
Benzalkonium chloride 0.1%
Antiseptic handwash
- For handwashing to decrease bacteria on the skin
-
For external use only
- In eyes
- if in eyes, rinse promptly and thoroughly with water
- discontinue use if irritation and redness develop
- skin irritation or redness occurs for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- wash hands to remove soil
- dispense palmful
- spread to cover hands, rub in well
- air dry, do not rinse or towel dry
- for additional information, see Material Safety Data Sheet (MSDS)
- for emergency medical information in USA call 1-800-391-1504
Inactive ingredients water (aqua), isopropyl alcohol, propylene glycol, FD&C Red 40, FD&C Blue 1
Call 1-800-532-7732
SSDC
NDC 63146-117-03
MYSTIC
Foaming Hand Sanitizer
Benzalkonium chloride 0.1%
Net Contents: 750 mL/25 US fl oz
Distributed by: SSDC Division, Kay Chemical Company
4050 Corporate Drive, #100
Grapevine, Texas 76051, USA 800.532.7732
Copyright, 2010 Kay Chemical Company All Rights Reserved
749436-01 - 749436/7301/0715
1112021
| MYSTIC FOAMING HAND SANITIZER
benzalkonium chloride solution |
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
| Labeler - Kay Chemical Company (003237021) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
