Lander Polar Ice
Dosage form: gel
Ingredients: Menthol 20mg in 1g
Labeler: Abaco Partners LLC DBA Surefil
NDC code: 20890-0020
Medically reviewed by Drugs.com. Last updated on Oct 8, 2024.
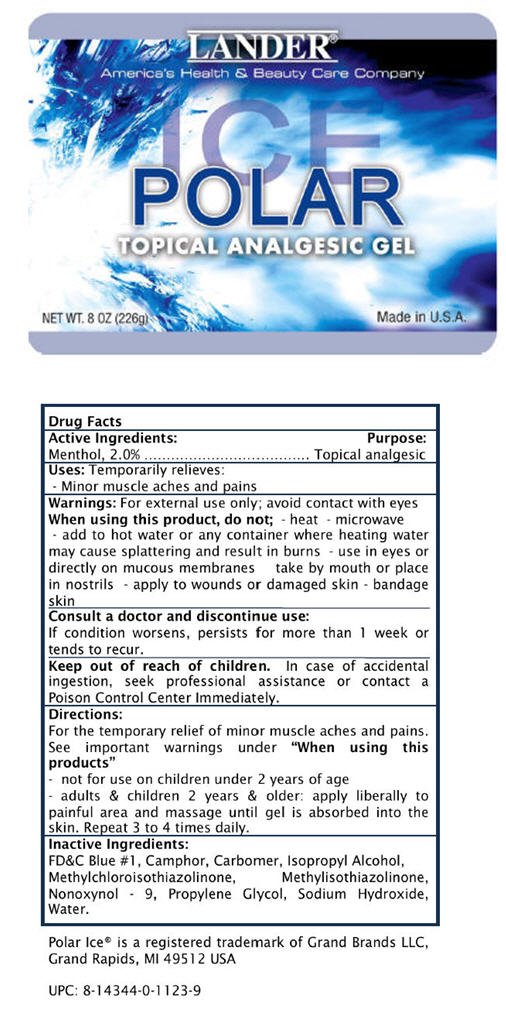
POLAR ICE
TOPICAL ANALGESIC GEL
Drug Facts
Menthol, 2.0%
Topical analgesic
Temporarily relieves:
- -
- Minor muscle aches and pains
For external use only; avoid contact with eyes
When using this product, do not;
- -
- heat
- -
- microwave
- -
- add to hot water or any container where heating water may cause splattering and result in burns
- -
- use in eyes or directly on mucous membranes
- take by mouth or place in nostrils
- -
- apply to wounds or damaged skin
- -
- bandage skin
Consult a doctor and discontinue use:
If condition worsens, persists for more than 1 week or tends to recur.
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center Immediately.
For the temporary relief of minor muscle aches and pains. See important warnings under "When using this products"
- -
- not for use on children under 2 years of age
- -
- adults & children 2 years & older: apply liberally to painful area and massage until gel is absorbed into the skin. Repeat 3 to 4 times daily.
FD&C Blue #1, Camphor, Carbomer, Isopropyl Alcohol, Methylchloroisothiazolinone, Methylisothiazolinone, Nonoxynol - 9, Propylene Glycol, Sodium Hydroxide, Water.
Polar Ice® is a registered trademark of Grand Brands LLC, Grand
Rapids, MI 49512 USA
UPC: 8-14344-0-1123-9
LANDER®
America's Health & Beauty Care Company
POLAR
ICE
TOPICAL ANALGESIC GEL
NET WT. 8 OZ (226g)
Made in U.S.A.
| LANDER
POLAR ICE
menthol gel |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Abaco Partners LLC DBA Surefil (964809417) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Anicare Pharmaceutical Pvt. | 916837425 | MANUFACTURE | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
