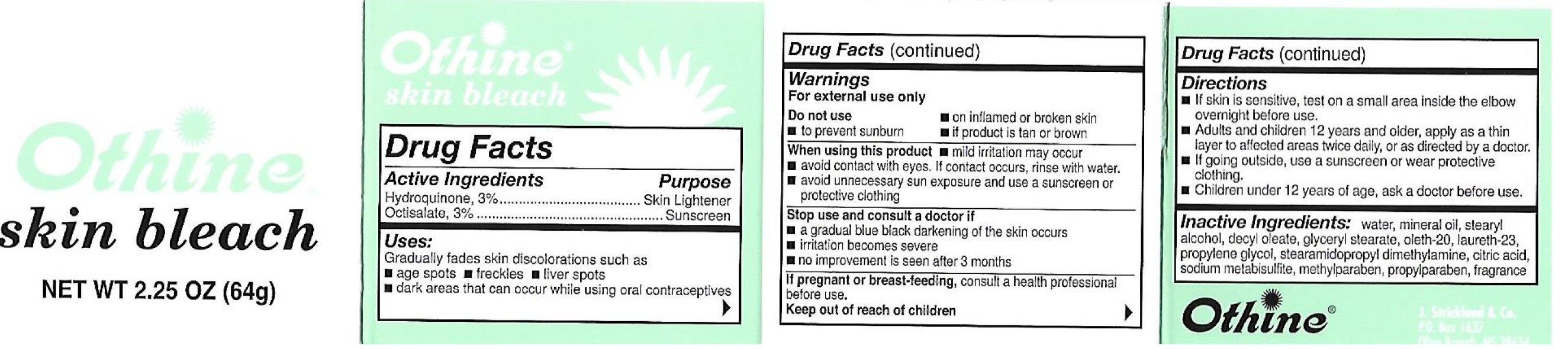
Othine Skin Bleach
Dosage form: cream
Ingredients: HYDROQUINONE 30mg in 1g, OCTISALATE 30mg in 1g
Labeler: J. Strickland & Co.
NDC code: 12022-016
Medically reviewed by Drugs.com. Last updated on Jan 9, 2025.
Active Ingredients
Hydroquinone, 3%; Octisalate, 3%
Purpose
Skin Lightener
Sunscreen
Uses:
Gradually fades areas of skin discoloration such as
- age spots
- freckles
- liver spots
- dark areas that can occur while using oral contraceptives.
Warnings:
For external use only
Do not use
- on inflamed or broken skin
- to prevent sunburn
- if product is tan or brown.
When using this product,
- mild irritation may occur
- avoid contact with eyes. If contact occurs, rinse with water.
- avoid unnecessary sun exposure and use a sunscreen or protective clothing
Stop use and consukt a doctor if
- a gradual blue-black darkening of the skin occurs
- irritation becomes severe
- no improvement is seen after 3 months
If pregnant or breast-feeding
consult a health professional before use.
Keep out of reach of children
consult a health professional before use.
Directions:
- If skin is sensitive, test on a small area inside elbow overnight before use.
- Adults and children 12 years and older, apply as a thin layer to affected area twice daily, or as directed by a doctor.
- If going outside, use a sunscreen or wear protective clothing
- Children under 12 years of age, ask a doctor before use.
Inactive Ingredients:
water, mineral oil, stearyl alcohol, decyl oleate, glyceryl stearate, oleth-20, laureth-23, propylene glycol, stearamidopropyl dimethylamine, citric acid, sodium metabisulfite, methylparaben, propylparaben, fragrance
Package Labeling
| OTHINE SKIN BLEACH
hydroquinone, octisalate cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - J. Strickland & Co. (007023112) |
| Registrant - J. Strickland & Co. (007023112) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| J. Strickland & Co. | 007023112 | manufacture(12022-016), pack(12022-016), label(12022-016) | |
Document Id: 6324462d-90c6-6012-e053-2991aa0a24de
Set id: 03e2bbac-f31a-407f-aa3e-639851c6c00a
Version: 1
J. Strickland & Co.
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
