CLINIQUE ANTIPERSPIRANT DEODORANT ROLL-ON
Dosage form: liquid
Ingredients: ALUMINUM CHLOROHYDRATE 70mL in 100mL
Labeler: CLINIQUE LABORATORIES INC
NDC code: 49527-571
Medically reviewed by Drugs.com. Last updated on Mar 31, 2025.
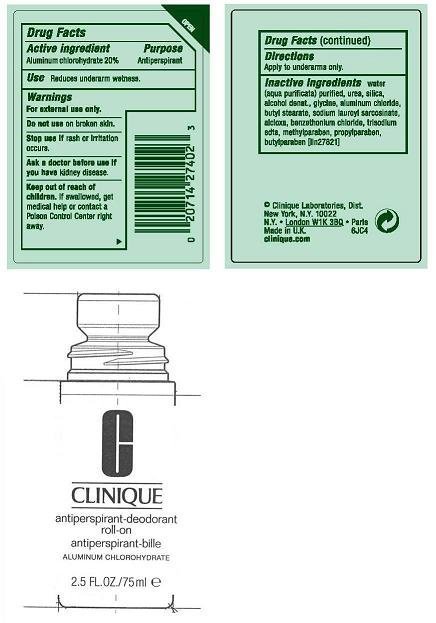
ACTIVE INGREDIENT:ALUMINUM CHLOROHYDRATE 20.00%
USES: DECREASES UNDERARM PERSPIRATION
WARNINGS:
- FOR EXTERNAL USE ONLY
- DO NOT USE ON BROKEN SKIN
- STOP USE IF RASH OR IRRITATION OCCURS
- ASK A DOCTOR BEFORE USE IF YOU HAVE KIDNEY DISEASE
KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
DIRECTIONS: APPLY TO UNDERARMS ONLY.
inactive ingredients: water[] urea [] silica [] alcohol denat. [] glycine [] aluminum chloride [] butyl stearate [] sodium lauroyl sarcosinate [] alcloxa [] benzethonium chloride [] trisodium edta [] methylparaben [] propylparaben [] butylparaben iln27821
PRINCIPAL DISPLAY PANEL:
CLINIQUE
anti-perspirant deodorant roll-on
ALUMINUM CHLOROHYDRATE
2.5FL OZ./ 70ML
CLINIQUE LABORATORIES, DIST.
NEW YORK, NY 10022
6183
CLINIQUE.COM
| CLINIQUE ANTIPERSPIRANT
DEODORANT ROLL-ON
aluminum chlorohydrate liquid |
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| Labeler - CLINIQUE LABORATORIES INC (173047747) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| ESTEE LAUDER COSMETICS, LTD | 205952385 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| ESTEE LAUDER N.V. | 370151326 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Len-Ron Manufacturing Division of Aramis Inc. | 809771152 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Aramis Inc. | 042918826 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Northtec Bristol | 949264774 | manufacture, relabel, repack | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Northtec Keystone | 618107429 | manufacture, relabel, repack | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| PADC 1 | 110482184 | manufacture, relabel, repack | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Estee Lauder Pennsylvania Distribution Center 2 | 828534516 | manufacture, relabel, repack | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Estee Lauder Cosmetics, Ltd. | 255175580 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Estee Lauder Cosmetics, Ltd | 253616536 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Estee Lauder Cosmetics Distribution Center | 208579636 | manufacture, label, relabel | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Estee Lauder Kabushiki Kaisha | 712808195 | relabel, repack | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Whitman Laboratories Ltd. | 216866277 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Aveda Corporation | 071352058 | manufacture | |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
