Zinbryta: Package Insert / Prescribing Info
Package insert / product label
Generic name: daclizumab
Dosage form: injection, solution
Drug class: Interleukin inhibitors
Medically reviewed by Drugs.com. Last updated on Aug 16, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
- Medication Guide
Highlights of Prescribing Information
ZINBRYTA (daclizumab) injection, for subcutaneous use
Initial U.S. Approval: 2016
WARNING: HEPATIC INJURY INCLUDING AUTOIMMUNE HEPATITIS and OTHER IMMUNE-MEDIATED DISORDERS
See full prescribing information for complete boxed warning.
Hepatic Injury Including Autoimmune Hepatitis
- ZINBRYTA can cause severe liver injury, including autoimmune hepatitis and liver failure. Fatal cases have occurred. Obtain transaminase and bilirubin levels before initiation of ZINBRYTA. Monitor and evaluate transaminase and bilirubin levels monthly and up to 6 months after the last dose (2.3, 2.4, 5.1).
- ZINBRYTA is contraindicated in patients with pre-existing hepatic disease or hepatic impairment (4, 5.1).
Other Immune-Mediated Disorders
- Immune-mediated disorders including skin reactions, lymphadenopathy, immune-mediated colitis, and other immune-mediated disorders can occur with ZINBRYTA (5.2).
These conditions may require treatment with systemic corticosteroids or immunosuppressive medication (5.1, 5.2).
ZINBRYTA is available only through a restricted distribution program called the ZINBRYTA REMS Program (5.3).
Recent Major Changes
Indications and Usage for Zinbryta
ZINBRYTA is an interleukin-2 receptor blocking antibody indicated for the treatment of adult patients with relapsing forms of multiple sclerosis (MS). Because of its safety profile, the use of ZINBRYTA should generally be reserved for patients who have had an inadequate response to two or more drugs indicated for the treatment of MS. (1)
Zinbryta Dosage and Administration
Dosage Forms and Strengths
Contraindications
Warnings and Precautions
- Hypersensitivity Reactions: Risk of anaphylaxis and angioedema. Discontinue and do not re-start ZINBRYTA if anaphylaxis or other allergic reactions occur (5.4)
- Infections: Increased risk of infections. If serious infection develops, consider withholding ZINBRYTA until infection resolves (5.5)
- Depression and Suicide: Advise patients to immediately report symptoms of depression and/or suicidal ideation to their health care provider. Consider discontinuation if severe depression and/or suicidal ideation occur (5.6)
Adverse Reactions/Side Effects
The most common adverse reactions (incidence ≥5% and ≥2% higher incidence than comparator) reported for ZINBRYTA were nasopharyngitis, upper respiratory tract infection, rash, influenza, dermatitis, oropharyngeal pain, bronchitis, eczema and lymphadenopathy compared with AVONEX; and upper respiratory tract infection, depression, rash, pharyngitis, and increased alanine aminotransferase (ALT) compared with placebo (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Biogen at 1-800-456-2255 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Hepatotoxic Drugs: Evaluate potential for increased risk of hepatotoxicity with concomitant use (7.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2017
Full Prescribing Information
WARNING: HEPATIC INJURY INCLUDING AUTOIMMUNE HEPATITIS and OTHER IMMUNE-MEDIATED DISORDERS
-
Hepatic Injury Including Autoimmune Hepatitis
ZINBRYTA can cause severe liver injury, including autoimmune hepatitis and liver failure. Fatal cases have occurred. Liver injury, including autoimmune hepatitis and acute liver failure, can occur at any time during treatment with ZINBRYTA, with cases reported up to 5 months after the last dose of ZINBRYTA.
ZINBRYTA is contraindicated in patients with pre-existing hepatic disease or hepatic impairment [see Contraindications (4) and Warnings and Precautions (5.1)].
Prior to starting ZINBRYTA, obtain serum transaminases (ALT and AST) and bilirubin levels [see Dosage and Administration (2.3)].
Test transaminase levels and total bilirubin monthly and assess before the next dose of ZINBRYTA. Follow transaminase levels and total bilirubin monthly for 6 months after the last dose of ZINBRYTA. In case of elevation in transaminases or total bilirubin, treatment interruption or discontinuation may be required [see Dosage and Administration (2.4) and Warnings and Precautions (5.1)].
-
Other Immune-Mediated Disorders
In addition to autoimmune hepatitis, a variety of immune-mediated disorders including skin reactions, lymphadenopathy, and immune-mediated colitis, and other serious conditions can occur in patients treated with ZINBRYTA. Overall, serious immune-mediated disorders were observed in 5% of patients treated with ZINBRYTA [see Warnings and Precautions (5.2)]. - If a patient develops a serious immune-mediated disorder, consider stopping ZINBRYTA and refer the patient to a specialist to ensure comprehensive diagnostic evaluation and appropriate treatment.
Some patients required systemic corticosteroids or other immunosuppressant treatment for autoimmune hepatitis or other immune-mediated disorders and continued this treatment after the last dose of ZINBRYTA [see Warnings and Precautions (5.1, 5.2)].
Because of the risks of hepatic injury, including autoimmune hepatitis, and other immune-mediated disorders, ZINBRYTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the ZINBRYTA REMS Program [see Warnings and Precautions (5.3)].
1. Indications and Usage for Zinbryta
ZINBRYTA is indicated for the treatment of adult patients with relapsing forms of multiple sclerosis (MS). Because of its safety profile, the use of ZINBRYTA should generally be reserved for patients who have had an inadequate response to two or more drugs indicated for the treatment of MS.
2. Zinbryta Dosage and Administration
2.1 Dosing Information
The recommended dosage of ZINBRYTA is 150 milligrams injected subcutaneously once monthly [see Dosage and Administration (2.3, 2.4)].
Instruct patients to inject a missed dose as soon as possible but no more than two weeks late. After two weeks, skip the missed dose and take the next dose on schedule. Administer only one dose at a time.
2.2 Important Administration Instructions
ZINBRYTA is for subcutaneous use only.
Train patients in the proper technique for self-administering subcutaneous injections using the prefilled autoinjector or syringe.
Thirty minutes prior to injection, remove ZINBRYTA from the refrigerator to allow the drug to warm to room temperature. Do not use external heat sources such as hot water to warm ZINBRYTA. Do not place ZINBRYTA back into the refrigerator after allowing it to warm to room temperature [see How Supplied/Storage and Handling (16.2)].
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. ZINBRYTA is a colorless to slightly yellow, clear to slightly opalescent solution. Do not use ZINBRYTA if it is cloudy or there are visible particles.
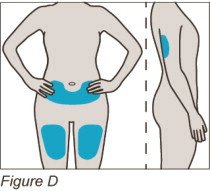
Sites for injection include the thigh, abdomen, and back of the upper arm.
Use each prefilled autoinjector or syringe one time and then place in a sharps disposal container for disposal according to community guidelines [see How Supplied/Storage and Handling (16.3)].
2.3 Assessment Prior to Initiating ZINBRYTA
Hepatic Assessment
Prior to initiating ZINBRYTA, obtain and evaluate the following:
- Serum transaminases (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)) and total bilirubin levels. Initiation of ZINBRYTA is contraindicated in patients with pre-existing hepatic disease or hepatic impairment including ALT or AST at least 2 times the ULN [see Contraindications (4) and Warnings and Precautions (5.1)].
Assessment for Tuberculosis and Other Infections
- Evaluate patients at high risk for tuberculosis infection prior to initiating treatment with ZINBRYTA [see Warnings and Precautions (5.5)]. For patients testing positive for tuberculosis, treat tuberculosis by standard medical practice prior to therapy with ZINBRYTA.
- Avoid initiating ZINBRYTA in patients with tuberculosis or other severe active infection [see Warnings and Precautions (5.5)].
- Prior to initiation of ZINBRYTA, screen patients for Hepatitis B and C. ZINBRYTA is contraindicated in patients with pre-existing hepatic disease [see Contraindications (4)].
Vaccinations
Because vaccination with live vaccines is not recommended during treatment and up to 4 months after discontinuation of treatment, consider any necessary immunization with live vaccines prior to treatment with ZINBRYTA [see Warnings and Precautions (5.5)].
2.4 Laboratory Testing and Monitoring to Assess Safety after Initiating ZINBRYTA
Conduct the following laboratory tests at periodic intervals to monitor for early signs of potentially serious adverse effects:
Liver Tests
Test transaminase levels and total bilirubin monthly and assess before the next dose of ZINBRYTA. Follow transaminase levels and total bilirubin monthly for 6 months after the last dose of ZINBRYTA. As shown in Table 1, interruption or discontinuation of ZINBRYTA therapy is recommended for management of certain liver test abnormalities [see Warnings and Precautions (5.1)].
| Elevated Transaminases and/or Total Bilirubin
[see Warnings and Precautions (5.1)] |
|
| Lab Value(s) | Recommendations |
| ALT or AST greater than 5 times ULN OR Total bilirubin greater than 2 times ULN OR ALT or AST greater than or equal to 3 but less than 5 times ULN and total bilirubin greater than 1.5 but less than 2 times ULN |
|
In clinical trials, permanent discontinuation of therapy was required if the patient had liver test abnormalities resulting in suspension of study treatment for at least 8 consecutive weeks.
ALT = alanine aminotransferase, AST = aspartate aminotransferase, ULN = upper limit of normal
3. Dosage Forms and Strengths
Injection: 150 mg/mL solution in a single-dose prefilled autoinjector.
Injection: 150 mg/mL solution in a single-dose prefilled syringe.
ZINBRYTA is a sterile, preservative-free, colorless to slightly yellow, clear to slightly opalescent solution.
4. Contraindications
ZINBRYTA is contraindicated in patients with:
- Pre-existing hepatic disease or hepatic impairment, including ALT or AST at least 2 times the ULN, because ZINBRYTA could exacerbate existing liver dysfunction [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
- A history of autoimmune hepatitis or other autoimmune condition involving the liver [see Warnings and Precautions (5.1)].
- A history of hypersensitivity to daclizumab or any other components of the formulation. Use in such patients may result in anaphylaxis or life-threatening multi-organ hypersensitivity [see Warnings and Precautions (5.4)].
5. Warnings and Precautions
5.1 Hepatic Injury
ZINBRYTA can cause severe liver injury, including autoimmune hepatitis and liver failure. Fatal cases have occurred. In controlled studies, serious drug-related hepatic injury occurred in 0.7% of ZINBRYTA-treated patients compared with 0.4% of AVONEX-treated patients (Study 1) and in 1.0% of ZINBRYTA-treated patients compared with no injury in placebo patients (Study 2). Across all clinical studies (controlled and open-label), serious drug-related hepatic injury occurred in 1.7% of ZINBRYTA-treated patients, with monthly monitoring of transaminases and total bilirubin. The incidence of discontinuation due to drug related hepatic injury was 5% in ZINBRYTA-treated patients and 4% in AVONEX-treated patients.
Across all clinical studies (controlled and open-label), 0.3% of ZINBRYTA-treated patients were diagnosed with autoimmune hepatitis. One fatal case of autoimmune hepatitis with acute liver failure occurred in a patient re-initiating ZINBRYTA after a planned 6 month treatment interruption period. This patient subsequently received two doses of ZINBRYTA in the presence of persisting alanine aminotransferase levels (ALT) more than 5 times the upper limit of normal (ULN).
Another case of acute liver failure occurred in a patient receiving ZINBRYTA in the postmarketing setting after 4 doses of ZINBRYTA, resulting in transplant and death. This patient had normal serum transaminase and total bilirubin levels 6 days prior to the last administration of ZINBRYTA. The patient was also receiving concomitant treatment with another drug known to be associated with hepatic injury [see Drug Interactions (7.1)].
Transaminase and Total Bilirubin Elevations
The incidence of increases in hepatic transaminases was greater in patients taking ZINBRYTA than in those taking AVONEX or placebo. The incidence of ALT or AST elevations above 5 times the ULN was 6% in ZINBRYTA-treated patients compared with 3% in AVONEX-treated patients (Study 1) and 4% in ZINBRYTA-treated patients compared with 1% in patients on placebo (Study 2). Less than 1% of ZINBRYTA-treated patients had ALT or AST greater than 20 times the ULN. Elevations of hepatic transaminases of at least 3 times the ULN combined with elevated bilirubin at least 2 times the ULN and alkaline phosphatase less than 2 times the ULN occurred in 0.7% of ZINBRYTA-treated patients compared with 0.1% of AVONEX-treated patients. In clinical trials, serum transaminase elevations occurred during treatment and up to 4 months after the last dose of ZINBRYTA.
Monitoring
Early identification of elevated liver enzymes may decrease the risk of a serious outcome. Prior to starting treatment with ZINBRYTA, obtain serum transaminases (ALT and AST) and total bilirubin levels [see Contraindications (4)].
Test transaminase levels and total bilirubin monthly and assess before the next dose of ZINBRYTA. Follow transaminase levels and total bilirubin monthly for 6 months after the last dose of ZINBRYTA.
Treatment modifications are recommended based on serum transaminase and total bilirubin values [see Dosage and Administration (2.4)].
Liver failure can occur at any time during treatment with ZINBRYTA even with monthly liver enzyme monitoring indicating normal values prior to each dose. Liver injury has been reported up to 5 months after the last dose of ZINBRYTA. Some cases of liver injury may be associated with fever, skin rash, or other immune-mediated disorders.
Monitor patients for signs and symptoms of hepatic injury. If a patient develops clinical signs or symptoms suggestive of hepatic dysfunction (e.g., unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine), promptly measure serum transaminases and total bilirubin and interrupt or discontinue treatment with ZINBRYTA, as appropriate.
Patients with prolonged elevations of serum transaminases should be evaluated for other possible causes, such as infection, and a specialist should evaluate the patient [see Dosage and Administration (2.4)]. Discontinue ZINBRYTA if autoimmune hepatitis is suspected. Treatment of autoimmune hepatitis with systemic corticosteroids and other immunosuppressant drugs may be required. Some patients may need long-term immunosuppression.
Risk of Hepatic Injury with Concomitant Use of Other Hepatotoxic Drugs
Caution should be used when using hepatotoxic drugs, including non-prescription products, concomitantly with ZINBRYTA. Also, carefully consider the need for the use of herbal products or dietary supplements that can cause hepatotoxicity [see Drug Interactions (7.1)].
5.2 Immune-Mediated Disorders
Treatment with ZINBRYTA increases the risk of immune-mediated disorders, including autoimmune disorders such as autoimmune hepatitis. Across all clinical studies (controlled and open-label), immune-mediated disorders occurred in 28% of patients on ZINBRYTA, the most common of which were skin reactions and lymphadenopathy. In the active-control study (Study 1), immune-mediated disorders were observed in 32% of ZINBRYTA-treated patients compared with 12% for AVONEX-treated patients. In Study 1, serious immune-mediated disorders were observed in 4% of patients treated with ZINBRYTA compared with less than 1% for AVONEX-treated patients. In the placebo-control study (Study 2), immune-mediated disorders were observed in 13% of ZINBRYTA-treated patients compared with 7% of placebo-treated patients. In Study 2, serious immune-mediated disorders were observed in 0.5% of ZINBRYTA-treated patients and in 0.5% of placebo-treated patients. In some cases, patients had concurrent or sequential occurring disorders while taking ZINBRYTA.
Some patients required invasive procedures for diagnosis (e.g., colonoscopy, liver biopsy, kidney biopsy, lung biopsy), hospitalization for fluid replacement or blood transfusion, or prolonged treatment with systemic corticosteroids or immunosuppressant drugs. Some of these events did not resolve after stopping ZINBRYTA during study follow-up.
Prescribers should be vigilant regarding emergent immune-mediated disorders. For suspected immune-mediated disorders, ensure adequate evaluation to confirm etiology or to exclude other causes. If a patient develops a serious immune-mediated disorder, consider stopping ZINBRYTA and refer the patient to an appropriate specialist for further evaluation and treatment.
Skin Reactions
ZINBRYTA causes skin reactions. In clinical trials, skin reactions occurred in 37% of ZINBRYTA-treated patients compared with 19% of AVONEX-treated patients (Study 1) and in 18% of ZINBRYTA-treated patients compared with 13% of patients on placebo (Study 2). Skin reactions occurred at any time during treatment with ZINBRYTA. Rashes occurred in 11% of ZINBRYTA-treated patients compared to 4% of AVONEX-treated patients, and in 7% of ZINBRYTA-treated patients compared to 3% of patients on placebo. Dermatitis occurred more frequently in ZINBRYTA-treated patients compared to AVONEX-treated patients or to patients on placebo, and eczema was observed more frequently in ZINBRYTA-treated patients compared to AVONEX-treated patients [see Adverse Reactions (6.1)]. Psoriatic conditions occurred in 2% of ZINBRYTA-treated patients compared with 0.3% of AVONEX-treated patients. Photosensitivity also occurred.
Serious skin reactions occurred in 2% of patients treated with ZINBRYTA compared with 0.1% of patients on AVONEX (Study 1) and in 1% of patients treated with ZINBRYTA compared with none treated with placebo (Study 2). One death resulted from infectious complications following a serious cutaneous reaction. In patients with a history of skin conditions, including eczema or psoriasis, use of ZINBRYTA may exacerbate those conditions. In addition to serious cases of dermatitis, eczema, psoriasis and drug eruptions, cases of erythema multiforme, erythema nodosum, exfoliative rash, and oral ulcers occurred in ZINBRYTA clinical trials. Treatment of skin reactions included treatment with topical or systemic corticosteroids or immunosuppressant drugs, including tacrolimus. In clinical trials, discontinuation because of skin reactions was 4% in ZINBRYTA-treated patients. Rashes took a mean of 3 months to resolve, some were unresolved at the time of the last evaluation.
If a patient develops a serious diffuse or inflammatory rash, it is recommended that a dermatologist evaluate the patient before the next dose of ZINBRYTA. Discontinuation of ZINBRYTA may be appropriate.
Lymphadenopathy
ZINBRYTA increases the incidence of lymphadenopathy. In controlled studies, lymphadenopathy or lymphadenitis occurred in 6% of ZINBRYTA-treated patients compared with 1% of AVONEX-treated patients (Study 1) and in 2% of ZINBRYTA-treated patients compared with 1% of placebo-treated patients (Study 2). Onset of lymphadenopathy or lymphadenitis occurred throughout the treatment period. Serious events related to lymphadenopathy or lymphadenitis included infections, benign salivary neoplasm, skin reactions, thrombocytopenia, and interstitial lung changes [see Warnings and Precautions (5.5)]. The majority of cases resolved with or without continued treatment with ZINBRYTA and took a mean of 3 months to resolve. Lymphadenopathy resulted in discontinuation in 0.6 % of ZINBRYTA-treated patients.
Some patients with lymphadenopathy underwent diagnostic biopsy. In the event that lymph node biopsy is considered, full diagnostic evaluation should be conducted by a specialist.
Autoimmune Hemolytic Anemia
Across all clinical studies (controlled and open-label), autoimmune hemolytic anemia occurred in <1% of patients treated with ZINBRYTA. Autoimmune hemolytic anemia resolved with discontinuation of ZINBRYTA, corticosteroid or other immunosuppressant treatment, and blood transfusions in most cases. If a patient develops signs or symptoms of autoimmune hemolytic anemia (e.g., pallor, fatigue, dark urine, jaundice, shortness of breath), consider discontinuing ZINBRYTA and referring to an appropriate specialist for further evaluation and treatment.
Immune-mediated Colitis
An increased incidence of serious colitis (less than 1%) was reported in patients treated with ZINBRYTA compared with none for patients treated with AVONEX or placebo in clinical trials. Cases have included reports of colitis, ulcerative colitis, Crohn's disease, microscopic colitis, inflammatory bowel disease, proctitis, and proctocolitis. Consider discontinuing ZINBRYTA and referring patients who develop symptoms of colitis (e.g., abdominal pain, fever, prolonged diarrhea, bloody stools) to a specialist.
Other immune-mediated disorders
A wide variety of other immune-mediated disorders, some serious, have occurred with the use of ZINBRYTA. These include single organ or systemic multi-organ inflammatory reactions [see Adverse Reactions (6.1)]. Some required treatment with systemic corticosteroids or other immunosuppressants. Some required several months for resolution after the last dose of ZINBRYTA, and some had not resolved even several months after discontinuation of ZINBRYTA, at the time of last reported follow-up.
For suspected immune-mediated disorders, ensure adequate evaluation to confirm etiology or to exclude other causes. If a patient develops a serious immune-mediated disorder, consider stopping ZINBRYTA and refer the patient to an appropriate specialist for further evaluation and treatment.
5.3 ZINBRYTA REMS Program
ZINBRYTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the ZINBRYTA REMS Program, because of the risks of hepatic injury including autoimmune hepatitis, and other immune-mediated disorders [see Warnings and Precautions (5.1, 5.2)].
Notable requirements of the ZINBRYTA REMS Program include the following:
- Prescribers must be certified with the program by enrolling and completing training.
- Patients must enroll in the program and comply with ongoing monitoring requirements [see Warnings and Precautions (5.1, 5.2)].
- Pharmacies must be certified with the program and must only dispense to patients who are authorized to receive ZINBRYTA.
Further information, including a list of qualified pharmacies/distributors, is available at 1-800-456-2255.
5.4 Acute Hypersensitivity
ZINBRYTA can cause anaphylaxis, angioedema, and urticaria after the first dose or at any time during treatment. Discontinue and do not re-start ZINBRYTA if anaphylaxis or other allergic reactions occur [see Contraindications (4)].
5.5 Infections
ZINBRYTA increases the risk for infections. In controlled trials, infections occurred in 65% of ZINBRYTA-treated patients compared with 57% of AVONEX-treated patients (Study 1) and in 50% of ZINBRYTA-treated patients compared with 44% of patients taking placebo (Study 2). Serious infections occurred in 4% of ZINBRYTA-treated patients compared with 2% of AVONEX-treated patients (Study 1) and in 3% of ZINBRYTA-treated patients compared with none on placebo (Study 2).
The most common types of infections observed were upper respiratory tract infections, urinary tract infections and viral infections.
Cytomegalovirus (CMV) infections (hepatitis and pneumonia) occurred in ZINBRYTA-treated patients in clinical trials.
In clinical trials, cases of tuberculosis occurred in countries where tuberculosis is endemic. Evaluate high-risk patients for tuberculosis infection prior to initiating treatment with ZINBRYTA. For patients testing positive for tuberculosis, treat by standard medical practice prior to therapy with ZINBRYTA [see Dosage and Administration (2.3)].
Avoid initiating ZINBRYTA in patients with severe active infection until the infection is fully controlled. If serious infection develops, consider withholding treatment with ZINBRYTA until the infection resolves.
Vaccinations
The safety of immunization with live viral vaccines during treatment with ZINBRYTA has not been studied. Vaccination with live vaccines is not recommended during treatment and up to 4 months after discontinuation of ZINBRYTA [see Dosage and Administration (2.3)].
5.6 Depression and Suicide
Depression-related events occurred more frequently in patients receiving ZINBRYTA than in patients receiving AVONEX or placebo. In controlled trials, depression-related events occurred in 10% of ZINBRYTA-treated patients compared with 8% of AVONEX-treated patients (Study 1) and in 7% of ZINBRYTA-treated patients compared with 2% of patients taking placebo (Study 2). In Study 1, serious events related to depression, including suicidal ideation or suicide attempt, occurred in 0.4% of ZINBRYTA-treated patients and in 0.7% of AVONEX-treated patients. None occurred in Study 2 (placebo-controlled).
Administer ZINBRYTA with caution to patients with previous or current depressive disorders. Advise patients and/or caregivers to immediately report any symptoms of new or worsening depression and/or suicidal ideation to their healthcare provider.
If a patient develops severe depression and/or suicidal ideation, consider discontinuation of ZINBRYTA.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described elsewhere in labeling:
- Hepatic Injury [see Warnings and Precautions (5.1)]
- Immune-Mediated Disorders [see Warnings and Precautions (5.2)]
- Acute Hypersensitivity [see Warnings and Precautions (5.4)]
- Infections [see Warnings and Precautions (5.5)]
- Depression and Suicide [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of ZINBRYTA cannot be directly compared with rates in clinical trials of other drugs and may not reflect the rates observed in practice.
In all controlled and uncontrolled trials performed in patients with relapsing multiple sclerosis, 2236 patients received ZINBRYTA for a total of 5214 person-years. Of these patients, 1576 received ZINBRYTA for at least 1 year, 1259 for at least 2 years, and 888 for at least 3 years. In the controlled studies, approximately 67% were female, 92% were Caucasian, and the mean age was 36 years at study entry.
In the active-controlled study (Study 1), 919 patients received ZINBRYTA (150 mg SQ, every 4 weeks) and 922 patients received AVONEX (interferon beta-1a 30 mcg IM, weekly) for a minimum of 2 years and up to 3 years, with 1952 person-years of exposure to ZINBRYTA; the median length of treatment was approximately 27 months. The adverse reactions from Study 1 are presented in Table 2.
In the placebo-controlled study (Study 2), 417 patients received ZINBRYTA with 423 person-years of exposure, of which 208 received 150 mg, and 204 received placebo every 4 weeks for up to 1 year; the median length of treatment was approximately 11 months. The adverse reactions from Study 2 are presented in Table 3.
The most common adverse reactions (incidence at least 5% and at least 2% higher incidence than comparator) that occurred in ZINBRYTA-treated patients were nasopharyngitis, upper respiratory tract infection, rash, influenza, dermatitis, oropharyngeal pain, bronchitis, eczema, and lymphadenopathy compared with AVONEX; and upper respiratory tract infection, depression, rash, pharyngitis, and increased alanine aminotransferase (ALT) compared with placebo.
The most common adverse reactions leading to discontinuation in up to 5% of patients treated with ZINBRYTA were hepatic events including elevations of serum transaminases and cutaneous events.
Patients were excluded from the clinical studies for abnormal laboratory values including hemoglobin, complete blood count with differential, serum transaminases, or serum creatinine. Patients were excluded if they had a history of seizure disorder or of having a seizure within 6 months of beginning the study, or suicidal ideation or severe depression within 3 months of beginning the study. During Study 1, concomitant use of ZINBRYTA with the hepatotoxic drugs valproic acid, carbamazepine, lamotrigine, phenytoin, isoniazid, and propylthiouracil was not permitted except in patients already receiving the drugs at the time of study entry.
In clinical studies, serum chemistry was evaluated at baseline and monthly. Hematology was evaluated at baseline, monthly for 6 months, and then every 3 months. Thyroid function was measured at baseline and every 6 months.
| Adverse Reaction | ZINBRYTA
150 mg SQ Every 4 Weeks N=919 % | AVONEX
30 mcg IM Once Weekly N=922 % |
|---|---|---|
|
1 includes upper respiratory tract infection and viral upper respiratory tract infection |
||
|
2 includes erythematous rash, exfoliative rash, macular rash, maculopapular rash, papular rash, pruritic rash, rash, and vesicular rash |
||
|
3 includes allergic dermatitis, atopic dermatitis, bullous dermatitis, dermatitis, exfoliative dermatitis, and seborrheic dermatitis |
||
|
4 includes dyshidrotic eczema, eczema, and nummular eczema |
||
| Nasopharyngitis | 25 | 21 |
| Upper respiratory tract infection 1 | 17 | 14 |
| Rash2 | 11 | 4 |
| Influenza | 9 | 6 |
| Dermatitis 3 | 9 | 2 |
| Oropharyngeal pain | 8 | 4 |
| Bronchitis | 7 | 5 |
| Eczema 4 | 5 | 2 |
| Lymphadenopathy | 5 | <1 |
| Tonsillitis | 4 | 2 |
| Acne | 3 | <1 |
| Adverse Reaction | ZINBRYTA
150 mg SQ Every 4 Weeks N=208 % | Placebo
N=204 % |
|---|---|---|
|
1 includes depressed mood and depression |
||
|
2 includes erythematous rash, exfoliative rash, macular rash, maculopapular rash, papular rash, pruritic rash, rash, and vesicular rash |
||
|
3 includes allergic dermatitis, atopic dermatitis, bullous dermatitis, dermatitis, exfoliative dermatitis, and seborrheic dermatitis |
||
| Upper respiratory tract infection | 9 | 7 |
| Depression1 | 7 | 2 |
| Rash2 | 7 | 3 |
| Pharyngitis | 6 | 4 |
| Increased ALT | 5 | 2 |
| Rhinitis | 4 | 1 |
| Anemia | 3 | <1 |
| Pyrexia | 3 | <1 |
| Increased AST | 3 | <1 |
| Dermatitis 3 | 3 | <1 |
Other clinically relevant adverse reactions observed at <2% difference from AVONEX or placebo in controlled trials included abnormal liver function test, colitis, decreased lymphocyte count, diarrhea, dry skin, erythema, folliculitis, increased hepatic enzyme, laryngitis, lymphadenopathy, pneumonia, pruritus, psoriasis, respiratory tract infection, skin exfoliation, toxic skin eruption, vasculitis and viral infection.
Seizures
In Study 1, seizures occurred in 1% of ZINBRYTA-treated patients, compared with 0.3% of AVONEX-treated patients. In Study 2, no seizures occurred in either treatment group.
Immune-mediated disorders
In addition to immune-mediated hepatitis, skin reactions, colitis, lymphadenopathy, and autoimmune hemolytic anemia, types of immune-mediated or autoimmune conditions that were observed in 2 or more ZINBRYTA-treated patients in controlled and open-label trials include immune cytopenias (agranulocytosis, thrombocytopenia, and pancytopenia), type I diabetes, celiac disease, immune-mediated thyroiditis, interstitial lung disease, lupus-like syndrome, pancreatitis, glomerulonephritis, rheumatoid arthritis, sarcoidosis, seronegative arthritis, sialadenitis, vasculitis, vitiligo, and multiorgan hypersensitivity [see Warnings and Precautions (5.2)].
Breast Cancer
In controlled studies, 1 ZINBRYTA-treated woman developed breast cancer compared with none in the AVONEX-treated group. Across all controlled and open-label clinical studies, 8 of 1485 (0.5%) ZINBRYTA-treated women developed breast cancer, and 1 of 751 (0.1%) ZINBRYTA-treated men developed breast cancer. It is unclear whether this represents an incidence increase over background rate.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. In Study 1, patients were tested for anti-drug (daclizumab) antibodies at Week 4 and approximately every 3 months thereafter. Anti-drug antibodies and neutralizing antibodies were observed in 19% (175/913) and 8% (71/913) of patients, respectively. Anti-drug antibody responses were transient in 12% (110/913) of patients and persistent in 7% (65/913) of patients. Anti-drug and neutralizing antibody responses predominantly occurred during the first year of treatment, and their frequency declined with continued ZINBRYTA treatment.
In patients with neutralizing antibodies, daclizumab clearance was increased on average by 19% [see Clinical Pharmacology (12.3)]. There was no apparent correlation of anti-drug antibody or neutralizing antibody development to clinical response, adverse reactions, or pharmacodynamic profile of ZINBRYTA.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to daclizumab with the incidence of antibodies to other products may be misleading.
Related/similar drugs
7. Drug Interactions
7.1 Hepatotoxic Drugs
Caution should be used when using hepatotoxic drugs, including non-prescription products, concomitantly with ZINBRYTA. Carefully consider the need for the use of herbal products or dietary supplements that can cause hepatotoxicity [see Warnings and Precautions (5.1)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with use of ZINBRYTA in pregnant women.
Administration of ZINBRYTA to monkeys during gestation resulted in embryofetal death and reduced fetal growth at maternal exposures greater than 30 times that expected clinically [see Data]. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
In monkeys administered ZINBRYTA (0, 10, 50, or 200 mg/kg) weekly by subcutaneous injection during organogenesis (gestation days 20 through 50), there was a decrease in fetal body weight and crown-rump length, and an increase in embryofetal death at the highest dose tested. Plasma exposure (AUC) at the no-effect dose of 50 mg/kg was approximately 30 times that in humans at the recommended human dose (RHD) of 150 mg.
In monkeys administered ZINBRYTA (50 mg/kg) weekly by subcutaneous injection from gestation day 50 to birth, there were no effects on pre- or postnatal development for up to 6 months after birth. Plasma exposure (AUC) at the administered dose was 55 times that in humans at the RHD.
8.2 Lactation
Risk Summary
There are no data on the presence of daclizumab in human milk, the effects on the breastfed child, or the effects of the drug on milk production. Daclizumab was excreted in the milk of ZINBRYTA-treated monkeys.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ZINBRYTA and any potential adverse effects on the breastfed child from ZINBRYTA or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of ZINBRYTA in patients less than 17 years old have not been established. Use of ZINBRYTA is not recommended in pediatric patients due to the risks of hepatic injury and immune-mediated disorders [see Warnings and Precautions (5.1, 5.2)].
8.5 Geriatric Use
Clinical studies of ZINBRYTA did not include a sufficient number of patients aged 65 and over to determine whether they respond differently than younger patients.
8.6 Hepatic Impairment
Clinical trials did not include patients with ALT or AST more than two times the ULN. Patients with signs and symptoms of hepatic impairment may be at increased risk for hepatotoxicity from ZINBRYTA [see Dosage and Administration (2.3, 2.4), Contraindications (4), and Warnings and Precautions (5.1)].
11. Zinbryta Description
Daclizumab is a humanized monoclonal antibody that binds to the alpha subunit of the interleukin-2 receptor (IL-2Rα, CD25). Daclizumab is composed of two humanized gamma-1 heavy chains and two humanized kappa light chains and has a molecular weight of approximately 144 kilodaltons (kDa).
ZINBRYTA injection is supplied as a sterile, preservative-free, colorless to slightly yellow, clear to slightly opalescent solution for subcutaneous use in a single-dose prefilled autoinjector and syringe. Each 1 mL prefilled autoinjector and syringe contains 150 mg daclizumab; polysorbate 80, USP (0.3 mg); sodium chloride (5.84 mg); sodium succinate, anhydrous (5.94 mg); succinic acid (0.35 mg); and Water for Injection, USP. The pH is 6.0.
12. Zinbryta - Clinical Pharmacology
12.1 Mechanism of Action
The precise mechanism by which daclizumab exerts therapeutic effects in multiple sclerosis is unknown but is presumed to involve modulation of IL-2 mediated activation of lymphocytes through binding to CD25, a subunit of the high-affinity IL-2 receptor.
12.2 Pharmacodynamics
During ZINBRYTA treatment, mean cell counts for the major immune subsets (T, B, and NK cells) remained within normal ranges. Total lymphocyte, T and B cell counts decreased less than 10% from baseline during the first year of treatment. Total lymphocyte counts returned to baseline levels approximately 8-12 weeks after the last dose of ZINBRYTA (150 mg).
12.3 Pharmacokinetics
The pharmacokinetics of ZINBRYTA are similar for healthy volunteers and patients with multiple sclerosis (MS).
Absorption
Following a single subcutaneous injection of ZINBRYTA, the maximum concentration occurred between 5 and 7 days. At steady state, daclizumab mean maximum serum concentration (Cmax) was 30 μg/mL, minimum serum concentration (Cmin) was 15 μg/mL, and area under the serum concentration-time curve over the dosing interval (AUCtau) values were approximately 640 μg-days per mL. The absolute bioavailability of 150 mg subcutaneous daclizumab was approximately 90%.
After administration of ZINBRYTA 150 mg subcutaneously every 4 weeks, serum daclizumab concentrations reached steady state by the fourth dose. Daclizumab accumulated to a level approximately 2.5-fold compared with a single dose.
The coefficient of variation between individual patients was approximately 35-40% for exposure (Cmax and AUC) and 27-51% for clearance and volume of distribution.
Distribution
In multiple sclerosis patients taking 150 mg subcutaneous doses of ZINBRYTA every 4 weeks, the estimated steady-state volume of distribution of daclizumab was approximately 6.34 liters.
Metabolism and Elimination
Because it is a protein, daclizumab is expected to undergo catabolism to peptides and amino acids in the same manner as endogenous IgG proteins without renal elimination. The estimated clearance of daclizumab is 0.212 liters per day with an elimination half-life of 21 days. Daclizumab clearance in patients who developed neutralizing antibodies was 19% higher [see Adverse Reactions (6.2)].
Specific Populations
Clinical studies did not identify significant differences in pharmacokinetic parameters between Japanese and Caucasian healthy volunteers. Covariate analyses did not identify significant differences in pharmacokinetic parameters based on gender, age, or weight for patients with relapsing forms of multiple sclerosis.
Drug Interaction Studies
ZINBRYTA 150 mg administered subcutaneously every 4 weeks for 12 weeks in patients with multiple sclerosis did not significantly affect the systemic exposure of concomitantly administered oral midazolam (CYP3A substrate), warfarin (CYP2C9 substrate), dextromethorphan (CYP2D6 substrate), omeprazole (CYP2C19 substrate), or caffeine (CYP1A2 substrate).
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Impairment of Fertility
ZINBRYTA (0, 10, 50, or 200 mg/kg) administered biweekly by subcutaneous injection to monkeys had no adverse effect on male (sperm motility, concentration, and morphology or testosterone levels) or female (estrus cycle length or estradiol/progesterone patterns) fertility endpoints. At the highest dose tested, plasma exposures (AUC) in males and females were 100 and 85 times, respectively, that at the recommended human dose (RHD) of 150 mg.
13.2 Animal Toxicology and/or Pharmacology
There was a dose-dependent increase in microglial aggregates in the brain and spinal cord of monkeys at subcutaneous doses greater than 10 mg/kg administered biweekly for up to 39 weeks. Microglial aggregates were, in some animals, associated with microhemorrhage; however, no evidence of neuronal injury was observed. There was evidence of reversibility by 12 weeks after the last dose.
14. Clinical Studies
The efficacy of ZINBRYTA was demonstrated in two randomized, double-blind, controlled studies (Study 1 and Study 2). Both studies evaluated 150 mg of subcutaneous ZINBRYTA taken once every four weeks in patients with relapsing multiple sclerosis (RMS).
Study 1: Active-Controlled Trial in RMS
Study 1 compared ZINBRYTA to 30 mcg weekly intramuscular doses of AVONEX in 1841 patients. The study included RMS patients who had either: 1) at least 2 relapses during the prior 3 years and at least one relapse in the year prior to randomization; or 2) one or more clinical relapses and one or more new T1 gadolinium (Gd)-enhancing or T2 hyperintense MRI lesions within the prior 2 years with at least one of these events in the prior 12 months. Patients with progressive forms of multiple sclerosis or an Expanded Disability Status Scale (EDSS) score greater than 5 were excluded. Treatment continued for up to 144 weeks until the last enrolled patient completed 96 weeks of treatment. Clinical assessments were to occur every 12 weeks and after relapse events. MRI scans were performed at Week 24 and Week 96.
The primary outcome measure of Study 1 was the annualized relapse rate (ARR). Additional outcome measures included the proportion of patients relapsed, the proportion of patients who experienced confirmed disability progression, and the number of new or newly enlarging T2 hyperintense lesions. Confirmed disability progression was defined as at least a 1 point increase from baseline EDSS (1.5 point increase for patients with baseline EDSS of 0) sustained for 12 weeks.
In Study 1, randomization assigned 919 patients to ZINBRYTA and 922 patients to AVONEX; 71% of ZINBRYTA- and 70% of AVONEX-treated patients completed at least 96 weeks of treatment with the assigned drug. At baseline, the mean age of patients was 36 years, the mean disease duration since diagnosis was 4.2 years, the mean EDSS score was 2.5, and the mean number of relapses in the prior year was 1.6. At baseline, 68% of patients were female, 46% of patients had MRI scans with T1 Gd-enhancing lesions and 41% of patients had previously taken one or more non-steroid treatments for MS.
ZINBRYTA had a statistically significant effect on the annualized relapse rate and on the number of new or newly enlarging T2 hyperintense lesions. There was no statistically significant effect on 12-week confirmed disability progression.
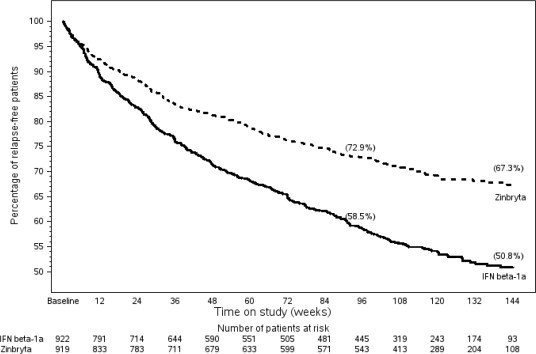
Results for Study 1 are shown in Table 4 and Figure 1, below.
| ZINBRYTA
150 mg SQ Every 4 Weeks N=919 | AVONEX
30 mcg IM Once Weekly N=922 | p-value | |
|---|---|---|---|
|
1Values refer to results up to144 weeks |
|||
|
2 MRI analysis used evaluable dataset and values reflect results at 96 weeks |
|||
| Clinical Results1 | |||
| Annualized relapse rate Relative reduction Proportion Relapse Free | 0.216 45% 67% | 0.393 51% |
<0.0001 |
| Proportion with 12-week confirmed disability progression | 16% | 20% | 0.16 |
| MRI Results2 | |||
| Mean number of new or newly enlarging T2 hyperintense lesions |
4.31 |
9.44 |
|
| Relative reduction | 54% | <0.0001 | |
Figure 1: Study 1 Percentage of Relapse-Free Patients
In a subgroup analysis of Study 1, a reduction was observed compared with AVONEX on annualized relapse rate across patient subgroups (based on gender, age, prior multiple sclerosis disease modifying therapy, and disease activity levels).
Study 2: Placebo-Controlled Trial in RMS
Study 2 compared ZINBRYTA to placebo in 412 patients. The study included RMS patients who had experienced at least one relapse in the year prior to randomization or who had one or more T1 Gd-enhancing MRI lesions within 6 weeks of randomization. Patients with progressive forms of multiple sclerosis or an EDSS score greater than 5 were excluded. Treatment duration was 52 weeks. Clinical assessments were to occur every 12 weeks and after relapse events. MRI scans were performed at weeks 24, 36, and 52 in all patients and every 4 weeks in a subset of patients.
The primary outcome measure of Study 2 was the annualized relapse rate (ARR) at Week 52. Additional outcome measures included new T1 Gd-enhancing lesions between Week 8 and Week 24, the proportion of patients relapsed, the proportion of patients who experienced 12-week confirmed disability progression (as defined in Study 1) and the number of new or newly enlarging T2 hyperintense lesions.
In Study 2, randomization assigned 208 patients to receive ZINBRYTA (150 mg) and 204 patients to receive placebo; 91% of ZINBRYTA patients and 91% of placebo patients completed treatment. At baseline, the mean age of patients was 36 years, the mean disease duration since diagnosis was 4.3 years, the mean EDSS score was 2.8, and the mean number of relapses in the prior year was 1.4. At baseline, 65% of patients were female, 48% of patients had MRI scans with T1 Gd-enhancing lesions and 21% of patients had previously taken one or more non-steroid treatments for MS.
ZINBRYTA had a statistically significant effect on the annualized relapse rate, the proportion of patients relapse free, the number of new T1 Gd-enhancing lesions, and the number of new or newly enlarging T2 hyperintense lesions. The results for Study 2 are shown in Table 5.
| ZINBRYTA
150 mg SQ Every 4 Weeks N=208 |
Placebo N=204 |
p-value |
|
|---|---|---|---|
|
1 Values refer to results at 52 weeks |
|||
|
2 MRI analyses used evaluable dataset for each endpoint; T1 Gd-enhancing: MRI intensive population, between 8-24 weeks |
|||
|
3 The proportion of patients with 12-week confirmed disability progression was an exploratory measure in Study 2. As the proportion of patients with 12-week confirmed disability was used as a key secondary outcome in Study 1, and is one of the main outcome measures in MS studies, the disability progression results are presented for Study 2. The nominal p value for that comparison, p=0.02, is not adjusted for multiple comparisons. |
|||
| Clinical Results1 | |||
| Annualized relapse rate Relative reduction Proportion Relapse Free | 0.211 54% 81% | 0.458 64% |
<0.0001 |
| Proportion with 12-week confirmed disability progression3
Relative risk reduction |
6% 57% |
13% |
|
| MRI Results2 | |||
| Mean number of new or newly enlarging T2 hyperintense lesions1 |
2.4 |
8.1 |
|
| Relative reduction | 70% | <0.0001 | |
| Mean number of new T1 Gd-enhancing lesions |
1.46 |
4.79 |
|
| Relative reduction | 69% | <0.0001 | |
16. How is Zinbryta supplied
16.1 How Supplied
ZINBRYTA injection is a sterile, colorless to slightly yellow, clear to slightly opalescent solution for subcutaneous injection. ZINBRYTA is provided in a 29 gauge, 0.5 inch staked (pre-affixed) needle syringe with a rubber plunger stopper and rigid needle shield that are not made with natural rubber latex.
Two presentations are provided for ZINBRYTA injection, a single-dose prefilled autoinjector and a single-dose prefilled syringe.
16.2 Storage and Handling
Store in a refrigerator between 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not freeze or expose to temperatures above 30°C (86°F). Discard if frozen.
If refrigeration is unavailable, ZINBRYTA may be stored protected from light up to 30°C (86°F) for a period up to 30 days. Do not place ZINBRYTA back into the refrigerator after allowing it to warm to room temperature. Discard after 30 days without refrigeration.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Hepatic Injury
Inform the patient of the risk of severe hepatic injury associated with ZINBRYTA. Advise patients of the symptoms of hepatic dysfunction, and instruct patients to report such symptoms to their health care provider immediately [see Warnings and Precautions (5.1)].
Discuss with the patient the importance of measuring hepatic laboratory values and having them evaluated by the health care provider monthly while taking ZINBRYTA and for up to 6 months after the last dose of ZINBRYTA.
Discuss with the patient the risk of concomitant use of other hepatotoxic medications, over the counter medications, herbal products, or dietary supplements.
Inform the patient that they will be given a ZINBRYTA Patient Wallet Card that they should carry with them at all times. This card describes symptoms which, if experienced, should prompt the patient to immediately seek medical evaluation.
Advise the patient to show the ZINBRYTA Patient Wallet Card to other treating health care providers.
Immune-Mediated Disorders
Advise patients that ZINBRYTA can cause their immune system to attack healthy cells in their body and that this can affect not only the liver but any organ system.
Skin Reactions
Advise patients that ZINBRYTA can cause dermatologic reactions that can range from mild rashes to serious reactions that could require treatment with other medications or result in hospitalization. Instruct patients to seek immediate medical attention if dermatologic reactions occur [see Warnings and Precautions (5.2)].
Lymphadenopathy
Inform patients that ZINBRYTA may cause lymphadenopathy that can range from mild events that can resolve on their own to serious lymphadenopathy that may require invasive procedures for diagnosis. Inform patients of the symptoms and instruct patients to contact their health care provider if they develop lymphadenopathy [see Warnings and Precautions (5.2)].
Autoimmune Hemolytic Anemia
Inform patients that ZINBRYTA may cause autoimmune hemolytic anemia that may be serious and could require blood transfusions and additional treatment. Advise patients of the symptoms of autoimmune hemolytic anemia and instruct patients to promptly contact their healthcare provider if they experience these symptoms [see Warnings and Precautions (5.2)].
Immune-mediated Colitis
Inform patients that ZINBRYTA may cause gastrointestinal reactions that may be serious and could require treatment. Advise patients of the symptoms of colitis and instruct patients to promptly contact their healthcare provider if they experience these symptoms [see Warnings and Precautions (5.2)].
ZINBRYTA REMS Program
ZINBRYTA is available only through a restricted program called the ZINBRYTA REMS Program [see Warnings and Precautions (5.3)]. Inform the patient of the following notable requirements:
- Patients must enroll in the program and comply with ongoing monitoring requirements [see Warnings and Precautions (5.1, 5.2)].
ZINBRYTA is available only from certified pharmacies participating in the program. Therefore, provide patients with the telephone number and website for information on how to obtain the product.
Allergic Reactions and Anaphylaxis
Advise patients of the symptoms of allergic reactions and anaphylaxis, and instruct patients to
seek immediate medical attention if these symptoms occur [see Warnings and Precautions (5.4)].
Risk of Infections
Inform patients that they may be more likely to get infections when taking ZINBRYTA, and that they should contact their health care provider if they develop symptoms of infection [see Warnings and Precautions (5.5)].
Depression and Suicide
Advise patients of the symptoms of depression and suicidal ideation as they have occurred with the use of ZINBRYTA and instruct patients to report symptoms of depression or thoughts of suicide to their health care provider immediately [see Warnings and Precautions (5.6)].
Instructions for Self-Injection Technique and Procedures
Provide appropriate instruction for methods of self-injection, including careful review of the ZINBRYTA Instructions for Use. Instruct the patient in the use of aseptic technique when administering ZINBRYTA.
Inform the patient that a health care provider should show them or their caregiver how to inject ZINBRYTA before administering the first dose. Tell the patient not to re-use needles, pens or syringes, and instruct the patient on safe disposal procedures. Inform the patient to dispose of used needles, pens and syringes in a puncture-resistant container.
46353-03
Manufactured by:
Biogen Inc.
Cambridge, MA 02142
U.S. License # 1697
Distributed by:
AbbVie Inc.
North Chicago, IL 60064
ZINBRYTA is a registered trademark of Biogen.
© 2016-2017 Biogen
|
This Medication Guide has been approved by the U.S. Food and Drug Administration Issued: 08/2017 |
|
| Medication Guide ZINBRYTA® (zin-bry-tuh) (daclizumab) injection, for subcutaneous use |
|
|
What is the most important information I should know about ZINBRYTA?
|
|
|
|
|
|
Because of the risk of serious liver problems (including autoimmune-related liver problems) and other immune system problems, ZINBRYTA is only available through a restricted program called the ZINBRYTA Risk Evaluation and Mitigation (REMS) Program.
|
|
| What is ZINBRYTA?
ZINBRYTA is a prescription medicine used to treat adults with relapsing forms of multiple sclerosis (MS). Because of its risks, ZINBRYTA is generally used in people who have tried 2 or more MS medicines that have not worked well enough. It is not known if ZINBRYTA is safe and effective for use in children under 18 years of age. |
|
Do not use ZINBRYTA if you:
|
|
Before using ZINBRYTA, tell your healthcare provider about all of your medical conditions, including if you:
|
|
| Tell your health care provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Using ZINBRYTA with other medicines can cause serious side effects. | |
How should I use ZINBRYTA?
|
|
| Your healthcare provider will decide which one is best for you. See the Instructions for Use that come with ZINBRYTA for detailed instructions on using ZINBRYTA the right way. Always use a new, unopened, single-dose prefilled autoinjector or single-dose prefilled syringe for each injection. Throw away any unused medicine. Do not re-use any syringes, needles or pens. |
|
| What are the possible side effects of ZINBRYTA?
ZINBRYTA can cause serious side effects, including:
|
|
|
|
|
|
|
|
| The most common side effects of ZINBRYTA include: | |
|
|
| Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of ZINBRYTA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store ZINBRYTA?
|
|
| Keep ZINBRYTA and all medicines out of the reach of children. | |
| General Information about the safe and effective use of ZINBRYTA.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ZINBRYTA for a condition for which it was not prescribed. Do not give ZINBRYTA to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ZINBRYTA that is written for health professionals. For more information, go to zinbryta.com or call 1-800-456-2255. |
|
| What are the ingredients in ZINBRYTA?
Active ingredient: daclizumab Inactive ingredients:
|
|
| Manufactured by: Biogen Inc., Cambridge, MA 02142; Distributed by: AbbVie Inc., North Chicago, IL 60064 ZINBRYTA is a registered trademark of Biogen. ©2016-2017 Biogen |
|
INSTRUCTIONS FOR USE
ZINBRYTA® (zin-bry-tuh)
(daclizumab)
Injection, for Subcutaneous Use
Single-Dose Prefilled Syringe
150 mg
Read this Instructions for Use before you start using ZINBRYTA and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Important information:
- Before you use ZINBRYTA for the first time, your healthcare provider should show you or your caregiver how to prepare and inject your ZINBRYTA prefilled syringe the right way.
- ZINBRYTA is for use under the skin only (subcutaneous).
- ZINBRYTA prefilled syringe is for 1 time use only. Do not share your ZINBRYTA prefilled syringe with other people. You may give an infection to them or get an infection from them.
- Throw away (dispose of) the ZINBRYTA prefilled syringe right away after use. See “Disposing of ZINBRYTA prefilled syringes” at the end of this Instructions for Use.
Supplies needed for your ZINBRYTA prefilled syringe injection (See Figure A):
- a ZINBRYTA 150 mg prefilled syringe
Additional supplies you will need for your injection (See Figure B):
- alcohol wipe
- gauze pad
- adhesive bandage
- 1 sharps container for throwing away ZINBRYTA prefilled syringes. See “Disposing of ZINBRYTA prefilled syringes” at the end of these instructions.
- a well-lit area and a clean, flat surface to work on, like a table
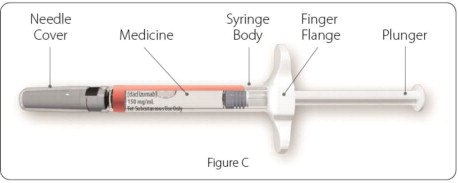
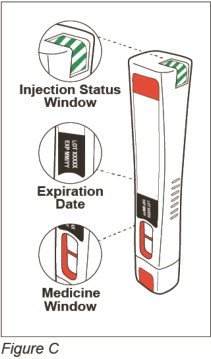
Parts of your ZINBRYTA prefilled syringe (See Figure C):
-
Before you prepare your injection, take your ZINBRYTA prefilled syringe out of the refrigerator and let it come to room temperature for at least 30 minutes.
- Do not use external heat sources such as hot water to warm the ZINBRYTA prefilled syringe.
- Do not use more than 1 prefilled syringe each month.
- The finger flange will allow you to hold the prefilled syringe more firmly. Do not remove the finger flange.
Preparing for your injection:
Step 1: Collect your supplies and wash your hands
- Find a well-lit and clean, flat surface, like a table and collect all the supplies you will need to give yourself or to receive an injection.
- Wash your hands with soap and water and dry your hands well.
| Step 2: Check your ZINBRYTA prefilled syringe | |
|
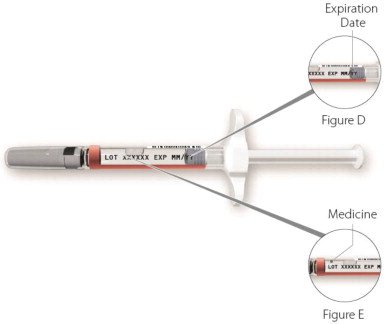 |
|
|
Giving your injection:
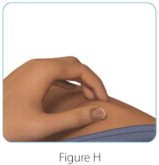
| Step 3: Choose and clean your injection site | |
|
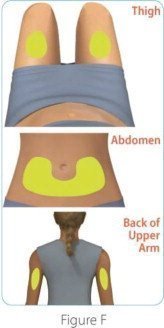 |
| Step 4: Remove the ZINBRYTA prefilled syringe needle cover | |
|
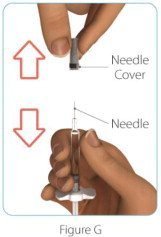 |
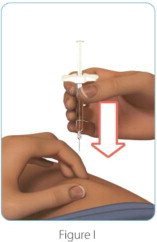
| Step 5: Give your ZINBRYTA prefilled syringe injection | |
|
 |
|
 |
|
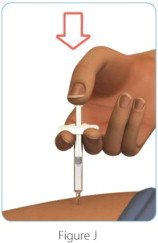 |
| Step 6: Remove the ZINBRYTA prefilled syringe from your injection site | |
|
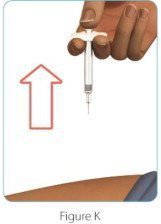 |
After your injection:
|
 |
How should I store ZINBRYTA Prefilled Syringe?
- Store ZINBRYTA prefilled syringe in the refrigerator between 36°F to 46°F (2°C to 8°C ).
- Do not freeze ZINBRYTA prefilled syringe or expose to temperatures above 86°F (30°C). Do not use ZINBRYTA prefilled syringe that has been frozen.
- Keep ZINBRYTA prefilled syringe in the original carton to protect it from light.
- If you cannot refrigerate ZINBRYTA prefilled syringe, you can store ZINBRYTA at room temperature for up to 30 days.
- If ZINBRYTA prefilled syringe has reached room temperature, do not put it back in the refrigerator.
- Keep ZINBRYTA prefilled syringe and all medicines out of the reach of children.
Questions?
For product or service related questions, please call 1-800-456-2255 or go to www.Zinbryta.com.
46352-03
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by: Biogen Inc., Cambridge, MA 02142
©2016-2017 Biogen. All rights reserved. 1-800-456-2255
Issued: 08/2017
INSTRUCTIONS FOR USE
ZINBRYTA®(zin-bry-tuh) PEN
(daclizumab)
Injection, for Subcutaneous Use
Single-Dose Prefilled Autoinjector
150 mg/mL
Do not remove the cap until you are ready to inject.
Read this Instructions for Use before you start using ZINBRYTA Pen and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Important information:
- Before you use the ZINBRYTA Pen for the first time, your healthcare provider should show you or your caregiver how to prepare and inject your ZINBRYTA Pen the right way.
- Each ZINBRYTA Pen is for 1 time use only.
- Do not share your ZINBRYTA Pen with other people. You may give an infection to them or get an infection from them.
- Do not inject more than 1 dose each month.
Do not use your ZINBRYTA Pen if it has been dropped or is visibly damaged. If you have dropped your ZINBRYTA Pen or it looks damaged, throw it away (dispose) and get a new one. See “Step 8: Dispose of your ZINBRYTA Pen”.
Supplies needed for your ZINBRYTA Pen injection (See Figures AandB below):
- 1 ZINBRYTA Pen (See Figure B)
- 1 alcohol wipe
- 1 gauze pad
- 1 adhesive bandage
- 1 sharps container for throwing away ZINBRYTA Pens. See “Step 8: Dispose of your ZINBRYTA Pen”
Before Use – Parts of your ZINBRYTA Pen (See Figure B)
Important: Do not remove the cap until you are ready to inject. If you remove the cap, do not recap the ZINBRYTA Pen. Recapping the ZINBRYTA Pen could cause it to lock.
Before you prepare your injection, take your ZINBRYTA Pen out of the refrigerator and let it sit at room temperature for at least 30 minutes.
- Do not use external heat sources such as hot water to warm the ZINBRYTA Pen.
Preparing for your injection
Step 1: Collect your supplies and wash your hands
- Find a well-lit area and a clean, flat surface, like a table, and collect all the supplies you will need to give yourself, or to receive an injection.
- Wash your hands with soap and water and dry your hands well.
| Step 2: Check your ZINBRYTA Pen (See Figure C) | |
|
 |
| Step 3: Choose and clean your injection site | |
|
 |
|
Giving Your Injection |
|
| Step 4: Remove the ZINBRYTA Pen cap | |
|
 |
| Step 5: Give your ZINBRYTA Pen injection | |
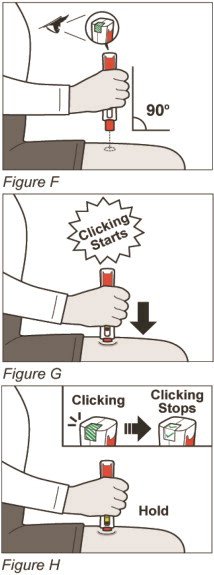
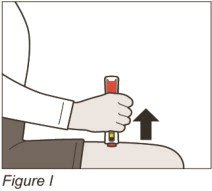
Firmly press and hold down your ZINBRYTA Pen on your injection site until you hear the clicking sounds start (See Figure G).
|
 |
| Step 6: Remove your ZINBRYTA Pen from your injection site | |
|
 |
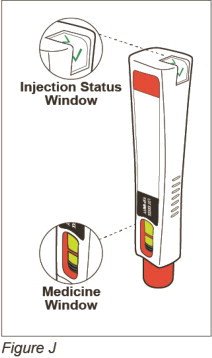
| Step 7: Check to make sure you have received your full dose of ZINBRYTA
(See Figure J) |
|
|
 |
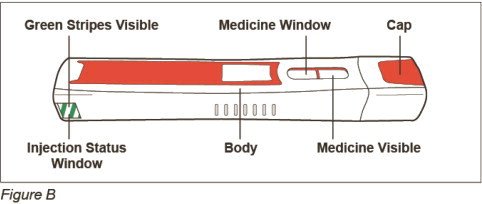
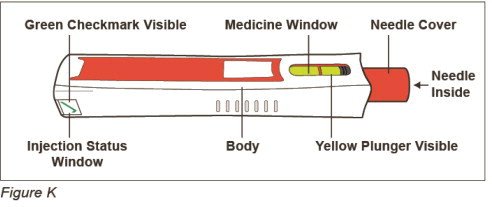
After Use – Parts of your ZINBRYTA Pen (See Figure K)
Note: After the pen has been removed from the injection site, the needle cover will lock to help prevent a needle stick injury. Do not recap your pen.
After your injection
|
 |
Step 9: Check your injection site
- If you see blood at your injection site, wipe it off with a gauze pad and apply an adhesive bandage.
How should I store ZINBRYTA?
- Store ZINBRYTA in the refrigerator between 36°F to 46°F (2°C to 8°C) in the closed original carton to protect it from light.
- Do not freeze ZINBRYTA or expose to temperatures above 86°F (30°C). Do not use ZINBRYTA that has been frozen.
- If you cannot refrigerate ZINBRYTA, you can store ZINBRYTA at room temperature in the closed original carton for up to 30 days.
- If ZINBRYTA has reached room temperature, do not put it back in the refrigerator.
- Keep ZINBRYTA and all medicines out of the reach of children.
For product or service related questions, please call 1-800-456-2255 or go to www.ZINBRYTA.com.
49006-01
This Instructions for Use has been approved by the U.S. Food and Drug Administration
Manufactured by: Biogen Inc., Cambridge, MA 02142
ZINBRYTA is a trademark of Biogen ©2016-2017 Biogen. All rights reserved. 1-800-456-2255
Issued: 05/2017
Principal Display Panel - Zinbryta Carton Label
NDC 0074-0033-01
Zinbryta®
(daclizumab)
Injection
Rx Only
150 mg/mL Single-Dose Prefilled Syringe
For Subcutaneous Use Only
Dispense with enclosed
medication guide
Once a Month
See package insert for
dosage and administration
Biogen® abbvie
Principal Display Panel - Zinbryta Syringe Label
Zinbryta®
(daclizumab)
150 mg/mL
Mfd by:
Biogen Inc.
For Subcutaneous Use Only
46351-02
Principal Display Panel - Zinbryta Pen Carton Label
NDC 0074-0034-01
Zinbryta®Pen
(daclizumab)
Injection
Rx Only
150 mg/mL Single-Dose
Prefilled Autoinjector
Contents: 1 Single-Dose Prefilled Autoinjector
For Subcutaneous Use Only
Dispense with enclosed
medication guide
Once a Month
See package insert for
dosage and administration
Biogen® abbvie
| ZINBRYTA
PEN
daclizumab injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ZINBRYTA
daclizumab injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Abbvie (078458370) |
Biological Products Related to Zinbryta
Find detailed information on biosimilars for this medication.
Frequently asked questions
More about Zinbryta (daclizumab)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: interleukin inhibitors
- Breastfeeding