P50 Pill: blue, capsule/oblong, 13mm
The pill with imprint P50 (Blue, Capsule/Oblong, 13mm) has been identified as Diphenhydramine Hydrochloride 50 mg and is used for Allergic Reactions, Insomnia, Allergic Rhinitis, Allergies, and Cold Symptoms. It belongs to the drug classes anticholinergic antiemetics, anticholinergic antiparkinson agents, antihistamines, miscellaneous anxiolytics, sedatives and hypnotics and is not a controlled substance.
Images for P50
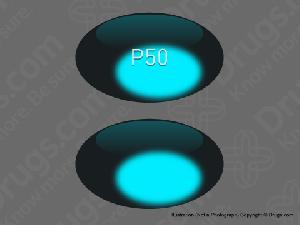
Diphenhydramine Hydrochloride
- Imprint
- P50
- Strength
- 50 mg
- Color
- Blue
- Size
- 13.00 mm
- Shape
- Capsule/Oblong
- Availability
- Rx and/or OTC
- Drug Class
- Anticholinergic antiemetics, Anticholinergic antiparkinson agents, Antihistamines, Miscellaneous anxiolytics, sedatives and hypnotics
- Pregnancy Category
- B - No proven risk in humans
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- P and L Development of New York Corporation
- National Drug Code (NDC)
- 59726-0230
- Inactive Ingredients
-
FD&C Blue No. 1,
gelatin,
glycerin,
polyethylene glycol,
water,
sorbitol
Note: Inactive ingredients may vary.
Submitted by: Drugs.com
Related images for "P50"
More about diphenhydramine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (548)
- Drug images
- Latest FDA alerts (2)
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: anticholinergic antiemetics
- Breastfeeding
Patient resources
Other brands
Benadryl, Banophen, Benadryl Allergy, ZzzQuil, ... +23 more
Professional resources
- DiphenhydrAMINE monograph
- Diphenhydramine (FDA)
- Diphenhydramine Capsules (FDA)
- Diphenhydramine Injection (FDA)
- Diphenhydramine Oral Solution (FDA)
Other brands
Benadryl, Benadryl Allergy, Diphen, Dicopanol
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.


