44-527 Pill: white, capsule/oblong
The pill with imprint 44-527 (White, Capsule/Oblong, 0mm) has been identified as Acetaminophen, Guaifenesin and Phenylephrine 325 mg / 200 mg / 5 mg and is used for Cough and Nasal Congestion. It belongs to the drug class upper respiratory combinations and is not a controlled substance.
Images for 44-527
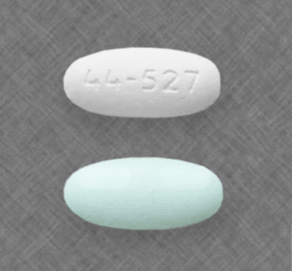
Acetaminophen, Guaifenesin and Phenylephrine
- Imprint
- 44-527
- Strength
- 325 mg / 200 mg / 5 mg
- Color
- White
- Shape
- Capsule/Oblong
- Availability
- Rx and/or OTC
- Drug Class
- Upper respiratory combinations
- Pregnancy Category
- N - Not classified
- CSA Schedule
- Not a controlled drug
- Labeler / Supplier
- LNK International Inc.
- National Drug Code (NDC)
Submitted by: pertalo
See also:
More about acetaminophen / guaifenesin / phenylephrine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Dosage information
- Drug class: upper respiratory combinations
Patient resources
Other brands
Theraflu Warming Relief Cold and Chest Congestion
Related treatment guides
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
