Hyaluronidase / Pertuzumab / Trastuzumab Dosage
Medically reviewed by Drugs.com. Last updated on Jul 3, 2025.
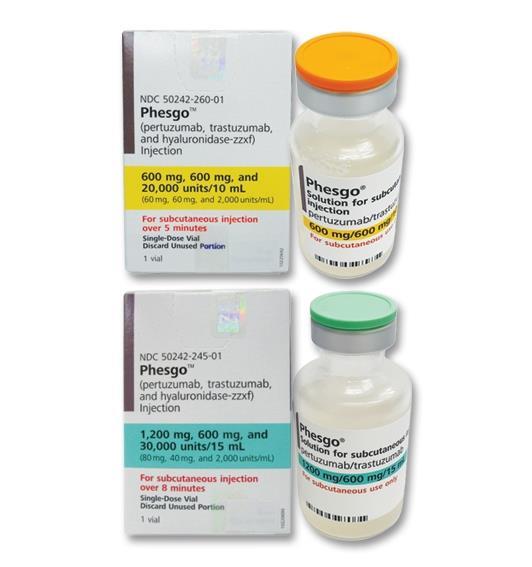
Applies to the following strengths: 30,000 units-1200 mg-600 mg/15 mL; 20,000 units-600 mg-600 mg/10 mL
Usual Adult Dose for:
Additional dosage information:
Usual Adult Dose for Breast Cancer
Initial dose: 1200 mg pertuzumab, 600 mg trastuzumab, and 30,000 units hyaluronidase subcutaneously over approximately 8 minutes
Maintenance dose: 600 mg pertuzumab, 600 mg trastuzumab, and 20,000 units hyaluronidase subcutaneously over approximately 5 minutes every 3 weeks after the initial dose
NOTE: Patients currently receiving IV pertuzumab and trastuzumab can transition to this combination. In patients receiving IV pertuzumab and trastuzumab with less than 6 weeks since their last dose, administer this combination as a maintenance dose of 600 mg pertuzumab/600 mg trastuzumab and every 3 weeks for subsequent administrations. In patients receiving IV pertuzumab and trastuzumab with 6 weeks or more since their last dose, administer this combination as an initial dose of 1200 mg pertuzumab/600 mg trastuzumab, followed by a maintenance dose of 600 mg pertuzumab/600 mg trastuzumab every 3 weeks for subsequent administrations.
Neoadjuvant Treatment of Breast Cancer:
- Administer this drug combination subcutaneously every 3 to 6 cycles as part of a treatment regimen for early breast cancer.
- Refer to the prescribing information for pertuzumab, administered in combination with trastuzumab and chemotherapy, for recommended dose and dose modifications.
- Following surgery, patients should continue to receive this drug combination to complete 1 year of therapy (up to 18 cycles) or until disease recurrence or unmanageable toxicity, whichever occurs first.
Adjuvant Treatment of Breast Cancer:
- Administer this drug combination every 3 weeks for a total of 1 year (up to 18 cycles) or until disease recurrence or unmanageable toxicity, whichever occurs first, as part of a complete regimen for early breast cancer, including standard anthracycline- and/or taxane-based chemotherapy.
- Start this drug combination on Day 1 of the first taxane-containing cycle.
Metastatic Breast Cancer (MBC):
- When administered with this drug combination, the recommended initial dose of docetaxel is 75 mg/m2 IV; the dose may be escalated to 100 mg/m2 every 3 weeks if the initial dose is well tolerated.
- Administer this drug combination until disease progression or unmanageable toxicity, whichever occurs first.
Uses:
- In combination with chemotherapy as a neoadjuvant treatment of patients with HER2-positive, locally advanced, inflammatory, or early stage breast cancer (either greater than 2 cm in diameter or node positive) as part of a complete treatment regimen for early breast cancer OR adjuvant treatment of patients with HER2-positive early breast cancer at high risk of recurrence
- Use in combination with docetaxel for treatment of patients with HER2-positive metastatic breast cancer (MBC) who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease
Renal Dose Adjustments
Data not available
Liver Dose Adjustments
Data not available
Dose Adjustments
DOSE MODIFICATIONS FOR LEFT VENTRICULAR DYSFUNCTION (LVEF):
EARLY BREAST CANCER:
- Pretreatment LVEF 55% or greater (for patients receiving anthracycline-based chemotherapy, a LVEF of 50% or greater is required after completion of anthracyclines, before starting this drug combination: Monitor LVEF every 12 weeks (once during neoadjuvant therapy).
- Withhold therapy for at least 3 weeks for an LVEF decrease to less than 50% with a fall of 10%-points or more below pretreatment value.
- Resume therapy after 3 weeks if LVEF has recovered EITHER to 50% or greater OR less than 10% points below pretreatment value.
- Pretreatment LVEF 50% or greater: Monitor LVEF every 12 weeks.
- Withhold therapy for at least 3 weeks for an LVEF decrease to EITHER less than 40% OR 20% to 45% with a fall of 10%-points or more below pretreatment value.
- Resume therapy after 3 weeks if LVEF has recovered to EITHER greater than 45% OR 40% to 45% with a fall of less than 10% points below pretreatment value.
Hypersensitivity and Administration-Related Reactions:
- Discontinue the injection immediately if the patient experiences a serious hypersensitivity reaction (e.g. anaphylaxis).
Precautions
US BOXED WARNINGS:
CARDIOMYOPATHY:
- This drug combination can cause subclinical and clinical cardiac failure.
- The incidence and severity are highest in patients receiving this drug combination with anthracycline-containing chemotherapy regimens.
- Evaluate cardiac function prior to and during therapy.
- Discontinue therapy in patients receiving adjuvant therapy and withhold this drug combination in patients with metastatic disease for clinically significant decrease in left ventricular function.
- This drug combination can cause embryofetal death and birth defects, including oligohydramnios and oligohydramnios sequence manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death.
- Advise patients of these risks and the need for effective contraception [see
- This drug combination can cause serious and fatal pulmonary toxicity.
- Discontinue therapy for anaphylaxis, angioedema, interstitial pneumonitis, or acute respiratory distress syndrome.
- Monitor patients until symptoms resolve.
CONTRAINDICATIONS:
- Hypersensitivity to the active component or any of the ingredients
Safety and efficacy have not been established in patients younger than 18 years.
Consult WARNINGS section for additional precautions.
Dialysis
Data not available
Other Comments
Administration advice:
- This drug combination is for subcutaneous use only in the thigh; do not administer IV.
- This drug combination has different dose and administration instructions than IV pertuzumab, IV trastuzumab, and subcutaneous trastuzumab when administered alone.
- Do not substitute this drug combination for or with pertuzumab, trastuzumab, ado-trastuzumab emtansine, or fam-trastuzumab deruxtecan.
- This drug combination should always be administered by a healthcare professional.
- In patients receiving an anthracycline-based regimen for early breast cancer, administer this drug combination following completion of the anthracycline.
- In patients receiving this drug combination for early breast cancer with docetaxel or paclitaxel, administer docetaxel or paclitaxel after this drug combination.
- In patients receiving this drug combination for metastatic breast cancer with docetaxel, administer docetaxel after this drug combination.
- Observe patients for a minimum of 30 minutes after the initial dose and 15 minutes after each maintenance dose for hypersensitivity administration-related reactions; medications to treat reactions, as well as emergency equipment, should be available for immediate use.
- For delayed or missed doses, if the time between 2 sequential injections is less than 6 weeks, administer the maintenance dose of 600 mg, 600 mg, and 20,000 units/10 mL; do not wait until the next planned dose.
- If the time between 2 sequential injections is 6 weeks or more, readminister the initial dose of 1200 mg, 600 mg, and 30,000 units/15 mL, followed every 3 weeks thereafter by a maintenance dose of 600 mg, 600 mg, and 20,000 units/10 mL.
Storage requirements:
- Store vials in the refrigerator at 2C to 8C (36F to 46F) in the original carton to protect from light.
- Do not freeze.
Reconstitution/preparation techniques:
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Do not use vial if particulates or discoloration is present.
- Do not shake.
- Discard any unused portion remaining in the vial.
- The manufacturer product information should be consulted.
Monitoring:
Assess left ventricular ejection fraction (LVEF) prior to initiation of therapy and at regular intervals during therapy.
More about hyaluronidase / pertuzumab / trastuzumab
- Check interactions
- Compare alternatives
- Reviews (1)
- Side effects
- During pregnancy
- Drug class: HER2 inhibitors
- En español
Patient resources
- Hyaluronidase, pertuzumab, and trastuzumab drug information
- Pertuzumab, trastuzumab, and hyaluronidase-zzxf (Advanced Reading)
Other brands
Professional resources
Other brands
Related treatment guides
See also:
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.