The originating document has been archived. We cannot confirm the completeness, accuracy, or currency of the content.
Tribrissen 48% Injection
This page contains information on Tribrissen 48% Injection for veterinary use.The information provided typically includes the following:
- Tribrissen 48% Injection Indications
- Warnings and cautions for Tribrissen 48% Injection
- Direction and dosage information for Tribrissen 48% Injection
Tribrissen 48% Injection
This treatment applies to the following species:Injection Sterile
For Use In Horses
Tribrissen 48% Injection Caution
Federal law restricts this drug to use by or on the order of a licensed veterinarian.Description
TRIBRISSEN 48% Injection is a sterile aqueous suspension of trimethoprim in a solution of the sodium salt of sulfadiazine for intravenous administration. Each mL contains trimethoprim 80 mg and sulfadiazine 400 mg.Vehicle Contains The Inactive Ingredients
diethanolamine 6 mg, sodium hydroxide 55 mg (additional may be added to adjust pH), polysorbate 80 0.2 mg, sodium metabisulfite 1 mg (at time of manufacture), and water for injection, q.s.TRIBRISSEN Injection is a combination of trimethoprim and sulfadiazine in the ratio of 1 part to 5 parts by weight, which provides effective antibacterial activity against a wide range of bacterial infections in animals.
Trimethoprim is 2,4 diamino-5(3,4,5-trimethoxybenzyl) pyrimidine.
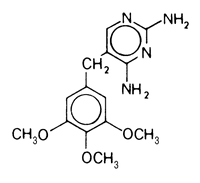
Actions
Microbiology: Trimethoprim blocks bacterial production of tetrahydrofolic acid from dihydrofolic acid by binding to and reversibly inhibiting the enzyme dihydrofolate reductase.Sulfadiazine, in common with other sulfonamides, inhibits bacterial synthesis of dihydrofolic acid by competing with para-aminobenzoic acid.
TRIBRISSEN Injection thus imposes a sequential double blockade on bacterial metabolism. This deprives bacteria of nucleic acids and proteins essential for survival and multiplication and produces a high level of antibacterial activity which is usually bactericidal.
Although both sulfadiazine and trimethoprim are antifolate, neither affects the folate metabolism of animals. The reasons are: animals do not synthesize folic acid and cannot, therefore, be directly affected by sulfadiazine; and although animals must reduce their dietary folic acid to tetrahydrofolic acid, trimethoprim does not affect this reduction because its affinity for dihydrofolate reductase of mammals is significantly less than for the corresponding bacterial enzyme.
TRIBRISSEN Injection is active against a wide spectrum of bacterial pathogens, both gram-negative and gram-positive. The following in vitro data are available, but their clinical significance is unknown. In general, species of the following genera are sensitive to TRIBRISSEN:
Very Sensitive
Escherichia, Streptococcus, Proteus, Salmonella, Pasteurella, Shigella, and Haemophilus.Sensitive
Staphylococcus, Neisseria, Klebsiella, Fusiformis, Corynebacterium, Clostridium, and Bordetella.Moderately Sensitive
Moraxella, Nocardia, and Brucella.Not Sensitive
Mycobacterium, Leptospira, Pseudomonas, and Erysipelothrix.As a result of the sequential double blockade of the metabolism of susceptible organisms by trimethoprim and sulfadiazine, the minimum inhibitory concentration (MIC) of TRIBRISSEN is markedly less than that of either of the components used separately. Many strains of bacteria that are not susceptible to one of the components are susceptible to TRIBRISSEN Injection. A synergistic effect between trimethoprim and sulfadiazine in combination has been shown experimentally both in vitro and in vivo (in dogs).
TRIBRISSEN Injection is bactericidal against susceptible strains and is often effective against sulfonamide-resistant organisms. In vitro sulfadiazine is usually only bacteriostatic.
Using a constant ration of one part trimethoprim in twenty parts of the combination, the following table shows MICs of bacteria which were susceptible to both trimethoprim (TMP) and sulfadiazine (SDZ). The organisms are those most commonly involved in conditions for which TRIBRISSEN is indicated.
Average Minimum Inhibitory Concentration (mic-mcg/ml) |
||||
|
Bacteria |
TMP Alone |
SDZ Alone |
TMP/SDZ |
|
|
TMP |
SDZ |
|||
|
Escherichia coli |
0.31 |
26.5 |
0.07 |
1.31 |
|
Proteus species |
1.3 |
24.5 |
0.15 |
2.85 |
|
Staphylococcus aureus |
0.6 |
17.6 |
0.13 |
2.47 |
|
Pasteurella species |
0.06 |
20.1 |
0.03 |
0.56 |
|
Salmonella species |
0.15 |
61.0 |
0.05 |
0.95 |
|
β Streptococcus |
0.5 |
24.5 |
0.15 |
2.85 |
The following table demonstrates the marked effect of the trimethoprim and sulfadiazine combination against sulfadiazine-resistant strains of normally susceptible organisms.
Average Minimum Inhibitory Concentration Of Sulfadiazine-resistant Strains (mic-mcg/ml) |
||||
|
Bacteria |
TMP Alone |
SDZ Alone |
TMP/SDZ |
|
|
TMP |
SDZ |
|||
|
Escherichia coli |
0.32 |
>245 |
0.27 |
5.0 |
|
Proteus species |
0.66 |
>245 |
0.32 |
6.2 |
The precise in vitro MIC of the combination varies with the ratio of the drugs present, but action of TRIBRISSEN Injection occurs over a wide range of ratios with an increase in the concentration of one of its components compensating for a decrease in the other. It is usual, however, to determine MICs using a constant ratio of one part trimethoprim in twenty parts of the combination.
Susceptibility Testing: In testing susceptibility to TRIBRISSEN Injection, it is essential that the medium used does not contain significant amounts of interfering substances which can bypass the metabolic blocking action, eg, thymidine or thymine.
The standard SxT disc is appropriate for testing by the disc diffusion method.
Pharmacology: Following parenteral administration, TRIBRISSEN Injection is rapidly absorbed and widely distributed throughout body tissues. Concentrations of trimethoprim are usually higher in tissues than in blood. The levels of trimethoprim are high in lung, kidney, and liver, as would be expected from its physical properties.
Serum concentration in horses following intravenous administration indicate rapid dissolution of trimethoprim particles and a steady rate of elimination of both components, with half-lives of about three hours and clearance within 24 hours.
Usually, the concentration of an antibacterial in the blood and the in vitro MIC of the infecting organism indicate an appropriate period between doses of a drug. This does not hold entirely for TRIBRISSEN Injection because trimethoprim, in contrast to sulfadiazine, localizes in tissues, and therefore, its concentration and ratio to sulfadiazine are higher there than in blood.
Serum levels following dosing give an indication, however, of the probable duration of effectiveness of a single dose.
The following table shows the average serum concentration of trimethoprim and sulfadiazine in 11 adult horses following administration of a single IV dose of 22 mg/kg.
Average Serum Concentration (mcg/ml) |
|||||||||
Trimethoprim (3.6 Mg/kg) |
Sulfadiazine (18 Mg/kg) |
||||||||
|
1 h |
3 h |
6 h |
8 h |
24 h |
1 h |
3 h |
6 h |
8 h |
24 h |
|
1.15 |
0.64 |
0.17 |
0.07 |
<0.02 |
27.2 |
16.4 |
7.5 |
4.5 |
0.09 |
Excretion of TRIBRISSEN is chiefly by the kidneys, by both glomerular filtration and tubular secretion. Urine concentrations of both trimethoprim and sulfadiazine are severalfold higher than blood concentrations. Neither trimethoprim nor sulfadiazine interferes with the excretion pattern of the other.
Tribrissen 48% Injection Indications And Usage
TRIBRISSEN therapy is indicated in horses where potent systemic antibacterial action against sensitive organisms is required. TRIBRISSEN 48% Injection is indicated where control of bacterial infections is required during treatment of: acute strangles, acute urogenital infections, respiratory tract infections, wound infections and abscesses. TRIBRISSEN is well tolerated by foals.Contraindications
TRIBRISSEN should not be used in horses showing marked liver parenchymal damage, blood dyscrasias, or in those with a history of sulfonamide sensitivity.Warning
Not for use in horses intended for human consumption.Adverse Reactions
Transient pruritis has been reported following the first dose in a small number of horses. This resolved spontaneously within 24 hours and did not recur after subsequent doses.Following administration intramuscularly, subcutaneously, or by accidental perivascular infiltration, swelling, pain, and minor tissue damage have occasionally be observed.
Serious, sometimes fatal, shock-like reactions accompanied by convulsions and collapse occurring within seconds to minutes following injection have been reported.
Individual animal hypersensitivity may result in local or generalized reactions, sometimes fatal. Anaphylactoid reactions, although rare, may also occur.
Antidote
Epinephrine.Post Approval Experience
Horses have developed diarrhea during TRIBRISSEN Injectable treatment, which could be fatal. If fecal consistency changes during TRIBRISSEN Injectable therapy, discontinue treatment immediately and contact your veterinarian.Precaution
Water should be readily available to horses receiving sulfonamide therapy. Rapid IV injection or excessive dosage may result in acute toxicity.Animal Safety
Toxicity is low. The acute toxicity (LD50) of TRIBRISSEN is more than 5 g/kg orally in rats and mice. No significant changes were recorded in rats given doses of 600 mg/kg per day for 90 days.Horses have tolerated up to five times the recommended daily dose for seven days or the recommended daily dose for 21 consecutive days without clinical effects or histopathological changes.
Lengthening of clotting time was seen in some of the horses on high or prolonged dosing in one of two trials. The effect, which may have been related to resolving infection, was not seen in a second similar trial.
Slight to moderate reductions in hematopoietic activity following high, prolonged dosage in several species have been recorded. This is usually reversible by folinic acid (leucovorin) administration or by stopping the drug. During long-term treatment of horses, periodic platelet counts and white and red blood cell counts are advisable.
Teratology
The effects of TRIBRISSEN 48% Injection on pregnancy has not been determined. Studies to date show there is no detrimental effect on stallion spermatogenesis with or following the recommended dose of TRIBRISSEN 48% Injection.Tribrissen 48% Injection Dosage And Administration
The recommended dose is 2 mL TRIBRISSEN 48% Injection per 100 lbs (45 kg) body weight per day. Shake well before using. Administer by intravenous injection. The usual course of treatment is a single, daily dose for 5 to 7 days. The daily dose may be halved and given morning and evening.
Continue acute infection therapy for two or three days after clinical signs have subsided.
A convenient dosage guide is:
250 lb body weight-5 mL daily
500 lb body weight-10 mL daily
750 lb body weight-15 mL daily
1000 lb body weight-20 mL daily
1250 lb body weight-25 mL daily
If no improvement of acute infections is seen in three to five days, reevaluate diagnosis.
TRIBRISSEN 48% Injection may be used alone or in conjunction with oral dosing. Following an initial injection, therapy can be maintained using TRIBRISSEN® 400 Oral Paste.
A complete blood count should be done periodically in patients receiving TRIBRISSEN for prolonged periods. If significant reduction in the count of any formed blood element should be noted, treatment with TRIBRISSEN should be discontinued.
How Supplied
TRIBRISSEN 48% Injection is available in 100 mL multiple dose vials.Store At 15º-30ºc (59º-86ºf).
Keep Out Of Reach Of Children.
NADA #106-965, Approved by FDA.
Manufactured for Schering-Plough Animal Health Corporation, Union, NJ 07083.
Copyright © 1998, Schering-Plough Animal Health Corp. All rights reserved.
|
|
NDC |
|
|
100 mL Multiple Dose Vial |
0061-5229-01 |
21337722 Rev. 4/04 |
Nac No.
10471992Distributed by INTERVET/SCHERING-PLOUGH ANIMAL HEALTH
29160 INTERVET LANE, P.O. BOX 318, MILLSBORO, DE, 19966-0318
| Toll-Free: | 800-992-8051 | |
| Customer Service: | 800-441-8272 | |
| Website: | www.intervetusa.com | |
| Email: | Information.USA@intervet.com |
 |
Every effort has been made to ensure the accuracy of the Tribrissen 48% Injection information published above. However, it remains the responsibility of the readers to familiarize themselves with the product information contained on the US product label or package insert. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
