Anigen Rapid Canine Parvovirus Antigen Test Kit, Blot Test
This treatment applies to the following species:One-step Canine Parvovirus Antigen Test
● Principles
The Canine Parvovirus Antigen Test Kit, Blot Test is a chromatographic immunoassay for the qualitative detection of Canine Parvovirus antigen in canine feces.
The Canine Parvovirus Antigen Test Kit, Blot Test has a letter “T” and “C” as test line and control line on the surface of the device. Both the test line and control line in the result window are not visible before applying any sample. The control line is used for procedural control. The control line should always appear if the test procedure is performed properly and the test reagents of the control line are working. A purple test line will be visible in the result window if there is Parvovirus antigen in the specimen.
The specially selected Canine Parvovirus antibodies are used in the test band as both capture and detector materials. These enable the Canine Parvovirus Antigen Test Kit, Blot Test to identify Canine Parvovirus antigens in canine feces.
● Materials provided (10 Tests/Kit)
1) Ten (10) Anigen Rapid Canine Parvovirus Antigen Test Devices
2) Ten (10) Specimen tubes containing 1 mL of assay diluent buffer (contains sodium azide as a preservative)
3) Ten (10) Sample collection swabs
4) Ten (10) Disposable droppers
5) One (1) Instructions for use
● Precautions
1) For veterinary diagnostic use only.
2) For best results, strict adherence to these instructions is required.
3) All specimens should be handled as being potentially infectious.
4) Do not open or remove test devices from their individually sealed pouches until immediately before their use.
5) Do not use the test device if the pouch is damaged or the seal is broken.
6) Do not reuse test device.
7) All reagents must be at room temperature (15-30°C) before running the assay.
8) Do not use reagents beyond the stated expiration date marked on the label.
9) The components in this kit have been quality control tested as standard batch unit.
10) Do not mix components from different lot numbers.
11) The assay diluent contains sodium azide, a hazardous chemical. The kit should be disposed according to local regulations for hazardous materials and in vitro diagnostics.
● Storage and Stability
The kit can be stored at room temperature or refrigerated (2-30°C). The test kit is stable through the expiration date marked on the package label. DO NOT FREEZE. Do not store the test kit in direct sunlight.
● Specimen Collection and Preparation
1) The samples from canine feces should be used for this test.
2) The specimens should be tested immediately as soon as the samples are collected.
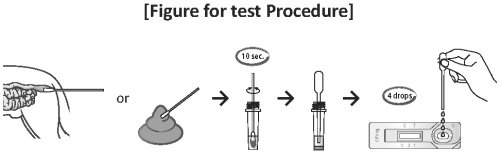
● Procedure of the Test
1) Collect the samples from canine feces using the swab.
2) Insert the swab into the specimen tube containing 1 mL of assay diluent.
3) Mix the swab samples with the assay diluent to extract well.
4) Remove the test device from the foil pouch, and place it on a flat and dry surface.
5) Use the disposable dropper provided to take the samples from extracted and mixed specimens in the tube.
6) Add four (4) drops into the sample hole using the disposable dropper. The mixed assay diluent should be added exactly, slowly, drop by drop.
7) As the test begins to work, you will see a purple color move across the result window in the center of the test device. If the migration has not appeared after 1 minute, add one more drop of the mixed assay diluent to the sample well.
8) Interpret test results at 5 - 10 minutes. Do not decide after 10 minutes.
● Interpretation of the Test
A color band will appear in the left section of the result window to show that the test is working properly. This band is the control band “C”. The right section of the result window indicates the test results. If another color band appears in the right section of the result window. This band is the test band “T”.
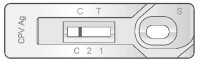
1) Negative Result
The presence of only one band “C” within the result window indicates a negative result.
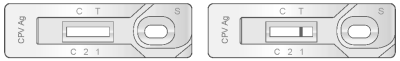
2) Positive Result
The presence of two color bands (“T” and “C”) within the result window, no matter which band appears first indicates a positive result.

3) Invalid Result
If the purple color band is not visible within the result window after performing the test, the result is considered invalid. The directions may not have been followed correctly or the test may have deteriorated. It is recommended that the specimen be retested.
● Limitations of the Test
When using the Canine Parvovirus Antigen Test Kit to detect Canine Parvovirus antigens, a low incidence of false results can occur. Other clinically available tests are required if questionable results are obtained. As with all diagnostic tests, a definitive clinical diagnosis should not be based on the results of a single test, but should only be made by the veterinarian after all clinical and laboratory findings have been evaluated.
● Key for symbols

Expiration date
Doc. No.: I1101-5E
Issued date: 19 Jan 2023
Manufactured by BioNote, Inc., 2-9, Seogu-dong, Hwaseong-si, Gyeonggi-do, Korea 445-170
Tel: +82-31-211-0516 / Fax: +82-31-8003-0618
bionote@bionote.co.kr / http://www.bionote.co.kr
Manufactured for
Modern Veterinary Therapeutics, LLC, 15491 SW 12 Street, Bldg D, Unit 403, Sunrise, Florida 33326 USA
Tel.: +1 407 852 8039 / Fax.: +1 305 503 8585
info@modernveterinarytherapeutics.com
http://www.modernveterinarytherapeutics.com
VPN 544B / PCN 5024.50
CPN: 1305005.5
15491 SW 12 STREET, UNIT 403, SUNRISE, FL, 33326
| Telephone: | 407-852-8039 | |
| Toll-Free: | 888-590-9839 | |
| Website: | www.modernveterinarytherapeutics.com | |
| Email: | info@modernveterinarytherapeutics.com |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". DVMetrics assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the DVMetrics service and data. See the Terms of Use for further details. |

Copyright © 2025 Animalytix LLC. Updated: 2025-08-27
