Zepzelca: Package Insert / Prescribing Info
Package insert / product label
Generic name: lurbinectedin
Dosage form: injection, powder, lyophilized, for solution
Drug class: Alkylating agents
J Code (medical billing code): J9223 (0.1 mg, injection)
Medically reviewed by Drugs.com. Last updated on Jul 7, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Drug Interactions
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- References
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
ZEPZELCA® (lurbinectedin) for injection, for intravenous use
Initial U.S. Approval: 2020
Indications and Usage for Zepzelca
ZEPZELCA is an alkylating drug indicated for the treatment of adult patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy. (1)
This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s). (1)
Zepzelca Dosage and Administration
- •
- Recommended Dosage: 3.2 mg/m2 every 21 days until disease progression or unacceptable toxicity. (2.1)
- •
- Administer ZEPZELCA as an intravenous infusion over 60 minutes. (2.1)
- •
- Moderate Hepatic Impairment: Recommended dosage is 1.6 mg/m2 every 21 days until disease progression or unacceptable toxicity. (2.4, 8.6)
- •
- Severe Hepatic Impairment: Avoid use of ZEPZELCA. If use cannot be avoided, the recommended dosage is 1.6 mg/m2 by intravenous infusion every 21 days until disease progression or unacceptable toxicity. (2.4, 8.6)
- •
- Consider premedication with corticosteroids and serotonin antagonists for antiemetic prophylaxis. (2.5)
Dosage Forms and Strengths
For injection: 4 mg lyophilized powder in a single-dose vial. (3)
Contraindications
None. (4)
Warnings and Precautions
- •
- Myelosuppression: Monitor blood counts prior to each administration. Initiate treatment with ZEPZELCA only if baseline neutrophil count is ≥ 1,500 cells/mm3 and platelet count is ≥ 100,000/mm3. Withhold, reduce the dose, or permanently discontinue ZEPZELCA based on severity. (2.2, 5.1)
- •
- Hepatotoxicity: Monitor liver function tests prior to initiating ZEPZELCA, periodically during treatment and as clinically indicated. Withhold, reduce the dose, or permanently discontinue ZEPZELCA based on severity. (2.2, 5.2)
- •
- Extravasation Resulting in Tissue Necrosis: Consider use of a central venous catheter to reduce the risk of extravasation. Monitor patients for signs and symptoms of extravasation during the ZEPZELCA infusion. If extravasation occurs, immediately discontinue the infusion, remove the infusion catheter, and monitor for signs and symptoms of tissue necrosis. (5.3)
- •
- Rhabdomyolysis: Monitor creatine phosphokinase (CPK) prior to initiating ZEPZELCA and periodically during treatment as clinically indicated. Withhold, reduce the dose, or permanently discontinue ZEPZELCA based on severity. (5.4)
- •
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females and males of reproductive potential of the potential risk to a fetus and to use an effective method of contraception. (5.5)
Adverse Reactions/Side Effects
The most common adverse reactions, including laboratory abnormalities, (≥20%) are leukopenia, lymphopenia, fatigue, anemia, neutropenia, increased creatinine, increased alanine aminotransferase, increased glucose, thrombocytopenia, nausea, decreased appetite, musculoskeletal pain, decreased albumin, constipation, dyspnea, decreased sodium, increased aspartate aminotransferase, vomiting, cough, decreased magnesium and diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Jazz Pharmaceuticals, Inc. at 1-800-520-5568 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- •
- Effect of Other Drugs on ZEPZELCA: Avoid coadministration. (7.1)
Use In Specific Populations
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2025
Full Prescribing Information
1. Indications and Usage for Zepzelca
ZEPZELCA is indicated for the treatment of adult patients with metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
This indication is approved under accelerated approval based on overall response rate and duration of response [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
2. Zepzelca Dosage and Administration
2.1 Recommended Dosage
The recommended dosage of ZEPZELCA is 3.2 mg/m2 by intravenous infusion over 60 minutes every 21 days until disease progression or unacceptable toxicity.
Initiate treatment with ZEPZELCA only if absolute neutrophil count (ANC) is at least 1,500 cells/mm3 and platelet count is at least 100,000/mm3.
2.2 Dosage Modifications for Adverse Reactions
The recommended dose reductions for adverse reactions are listed in Table 1.
Permanently discontinue ZEPZELCA in patients who are unable to tolerate 2 mg/m2 or require a dose delay greater than two weeks.
|
Dose Reduction |
Total Dose |
|
First Second |
2.6 mg/m2 every 21 days 2 mg/m2 every 21 days |
Dosage modifications for ZEPZELCA for adverse reactions are presented in Table 2.
| a National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0 | ||
| b Patients with isolated Grade 4 neutropenia (neutrophil count less than 500 cells/mm3) may receive G-CSF prophylaxis rather than undergo lurbinectedin dose reduction. | ||
|
Adverse Reaction |
Severitya |
Dosage Modification |
|
Neutropeniab |
Grade 4 or Any grade febrile neutropenia |
|
|
Thrombocytopenia |
Grade 3 with bleeding or Grade 4 |
|
|
Hepatotoxicity |
Grade 2 |
|
|
Grade ≥ 3 |
|
|
|
Rhabdomyolysis |
Grade 2 |
|
|
Grade ≥ 3 |
|
|
|
Other Adverse Reactions [see Postmarketing (6.2)] |
Grade 2 |
|
|
Grade ≥ 3 |
|
|
2.3 Dosage Modifications for Use with Strong and Moderate CYP3A Inhibitors
Avoid coadministration of ZEPZELCA with strong or moderate CYP3A inhibitors. If coadministration of ZEPZELCA with a strong or moderate CYP3A inhibitor cannot be avoided, reduce the dose of ZEPZELCA by 50%[see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. After discontinuation of a strong or moderate CYP3A inhibitor for 5 half-lives of the inhibitor, increase the ZEPZELCA dose to the dose used before starting the inhibitor.
2.4 Dosage Modifications for Patients with Severe and Moderate Hepatic Impairment
Avoid administration of ZEPZELCA in patients with severe hepatic impairment (total bilirubin > 3 × Upper Limit of Normal (ULN)). If administration of ZEPZELCA cannot be avoided, the recommended dosage is 1.6 mg/m2 by intravenous infusion over 60 minutes every 21 days until disease progression or unacceptable toxicity [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
In patients with moderate hepatic impairment (total bilirubin > 1.5 to ≤ 3 × ULN and any AST), the recommended dosage of ZEPZELCA is 1.6 mg/m2 by intravenous infusion over 60 minutes every 21 days until disease progression or unacceptable toxicity.
2.5 Premedication
Consider administering the following pre-infusion medications for antiemetic prophylaxis [see Adverse Reactions (6.1)]:
- •
- Corticosteroids (dexamethasone 8 mg intravenously or equivalent)
- •
- Serotonin antagonists (ondansetron 8 mg intravenously or equivalent)
2.6 Preparation, Administration and Storage
ZEPZELCA is a hazardous drug. Follow applicable special handling and disposal procedures1.
Preparation
- •
- Inject 8 mL of Sterile Water for Injection USP into the vial, yielding a solution containing 0.5 mg/mL lurbinectedin. Shake the vial until complete dissolution.
- •
- Visually inspect the solution for particulate matter and discoloration. The reconstituted solution is a clear, colorless or slightly yellowish solution, essentially free of visible particles.
- •
- Calculate the required volume of reconstituted solution as follows:
- •
- For administration through a central venous line, withdraw the appropriate amount of reconstituted solution from the vial and add to an infusion container containing at least 100 mL of diluent (0.9% Sodium Chloride Injection USP or 5% Dextrose Injection USP).
- •
- For administration through a peripheral venous line, withdraw the appropriate amount of reconstituted solution from the vial and add to an infusion container containing at least 250 mL of diluent (0.9% Sodium Chloride Injection USP or 5% Dextrose Injection USP).
Administration
- •
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If particulate matter is observed, do not administer.
- •
- ZEPZELCA can be administered with or without an in-line filter. If infusion lines containing in-line filters are utilized for administration of ZEPZELCA, polyethersulfone (PES) in-line filters with pore sizes of 0.22 micron are recommended.
- o
- Do not use in-line nylon membrane filters when the reconstituted ZEPZELCA solution is diluted using 0.9% Sodium Chloride Injection, USP. Adsorption of ZEPZELCA to the Nylon membrane filters has been observed when 0.9% Sodium Chloride Injection, USP is used as the diluent.
- •
- Compatibility with other intravenous administration materials and the diluted ZEPZELCA solution has been demonstrated in the following materials:
- o
- Polyolefin containers (polyethylene, polypropylene and mixtures).
- o
- Polyvinyl Chloride (PVC) (non-DEHP-containing), polyurethane and polyolefin infusion sets (polyethylene, polypropylene and polybutadiene).
- o
- Implantable venous access systems with titanium and plastic resin ports and with polyurethane or silicone intravenous catheters.
- •
- Do not co-administer ZEPZELCA and other intravenous drugs concurrently within the same intravenous line.
Storage of Infusion Solution
- •
- If not used immediately after reconstitution or dilution, the ZEPZELCA solution can be stored prior to administration for up to 24 hours following reconstitution, including infusion time, at either room temperature/ ambient light or under refrigeration at 2ºC to 8ºC (36ºF to 46ºF) conditions.
3. Dosage Forms and Strengths
For injection: 4 mg of lurbinectedin as a sterile, preservative-free, white to off-white lyophilized powder in a single-dose vial for reconstitution prior to intravenous infusion.
5. Warnings and Precautions
5.1 Myelosuppression
ZEPZELCA can cause myelosuppression.
In clinical studies of 554 patients with advanced solid tumors receiving ZEPZELCA [see Adverse Reactions (6.1)], Grade 3 or 4 neutropenia occurred in 41% of patients, with a median time to onset of 15 days and a median duration of 7 days. Febrile neutropenia occurred in 7% of patients. Sepsis occurred in 2% of patients and was fatal in 1% (all cases occurred in patients with solid tumors other than SCLC). Grade 3 or 4 thrombocytopenia occurred in 10%, with a median time to onset of 10 days and a median duration of 7 days. Grade 3 or 4 anemia occurred in 17% of patients.
Administer ZEPZELCA only to patients with baseline neutrophil count of at least 1,500 cells/mm3 and platelet count of at least 100,000/mm3. Monitor blood counts including neutrophil count and platelet count prior to each administration. For neutrophil count less than 500 cells/mm3 or any value less than lower limit of normal, the use of G-CSF is recommended. Withhold, reduce the dose, or permanently discontinue ZEPZELCA based on severity [see Dosage and Administration (2.2)].
5.2 Hepatotoxicity
ZEPZELCA can cause hepatotoxicity.
In clinical studies of 554 patients with advanced solid tumors receiving ZEPZELCA [see Adverse Reactions (6.1)], Grade 3 elevations of ALT and AST were observed in 6% and 3% of patients, respectively, and Grade 4 elevations of ALT and AST were observed in 0.4% and 0.5% of patients, respectively. The median time to onset of Grade ≥ 3 elevation in transaminases was 8 days (range: 3 to 49), with a median duration of 7 days.
Monitor liver function tests prior to initiating ZEPZELCA and periodically during treatment as clinically indicated. Withhold, reduce the dose, or permanently discontinue ZEPZELCA based on severity [see Dosage and Administration (2.2)].
5.3 Extravasation Resulting in Tissue Necrosis
Extravasation of ZEPZELCA resulting in skin and soft tissue injury, including necrosis requiring debridement, can occur. Consider use of a central venous catheter to reduce the risk of extravasation, particularly in patients with limited venous access. Monitor patients for signs and symptoms of extravasation during the ZEPZELCA infusion. If extravasation occurs, immediately discontinue the infusion, remove the infusion catheter, and monitor for signs and symptoms of tissue necrosis. The time to onset of necrosis after extravasation may vary.
Administer supportive care and consult with an appropriate medical specialist as needed for signs and symptoms of extravasation. Administer subsequent infusions at a site that was not affected by extravasation.
5.4 Rhabdomyolysis
Rhabdomyolysis has been reported in patients treated with ZEPZELCA. Monitor creatine phosphokinase (CPK) prior to initiating ZEPZELCA and periodically during treatment as clinically indicated. Withhold or reduce the dose based on severity [see Dosage and Administration (2.2)].
5.5 Embryo-Fetal Toxicity
Based on animal data and its mechanism of action ZEPZELCA can cause fetal harm when administered to a pregnant woman. Intravenous administration of a single dose of lurbinectedin (approximately 0.2 times the 3.2 mg/m2 clinical dose) to pregnant animals during the period of organogenesis caused 100% embryolethality in rats. Advise pregnant women of the potential risk to a fetus. Advise female patients of reproductive potential to use effective contraception during treatment with ZEPZELCA and for 6 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ZEPZELCA and for 4 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- Myelosuppression [see Warnings and Precautions (5.1)]
- •
- Hepatotoxicity [see Warnings and Precautions (5.2)]
- •
- Extravasation Resulting in Tissue Necrosis [see Warnings and Precautions (5.3)]
- •
- Rhabdomyolysis [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in the WARNINGS AND PRECAUTIONS reflects exposure to ZEPZELCA as a single agent at a dose of 3.2 mg/m2 intravenously every 21 days in 554 patients with advanced solid tumors. Among 554 patients who received ZEPZELCA, including 105 patients with small cell lung cancer (SCLC) in PM1183-B-005-14 (Study B-005), 24% were exposed for 6 months or longer and 5% were exposed for greater than one year.
Small Cell Lung Cancer (SCLC)
The safety of ZEPZELCA was evaluated in a cohort of 105 patients with previously treated SCLC in Study B-005 [see Clinical Studies (14)]. Patients received ZEPZELCA 3.2 mg/m2 intravenously every 21 days. All patients in this study received a pre-specified anti-emetic regimen consisting of a corticosteroid and serotonin antagonist. Patients could receive G-CSF for secondary prophylaxis (i.e., after patients had an initial decrease in WBC), but not primary prophylaxis. Among patients who received ZEPZELCA, 29% were exposed for 6 months or longer and 6% were exposed for greater than one year.
Serious adverse reactions occurred in 34% of patients who received ZEPZELCA. Serious adverse reactions in ≥ 3% of patients included pneumonia, febrile neutropenia, neutropenia, respiratory tract infection, anemia, dyspnea, and thrombocytopenia.
Permanent discontinuation due to an adverse reaction occurred in two patients (1.9%) who received ZEPZELCA. Adverse reactions resulting in permanent discontinuation in ≥ 1% of patients who received ZEPZELCA, which included peripheral neuropathy and myelosuppression.
Dosage interruptions due to an adverse reaction occurred in 30.5% of patients who received ZEPZELCA. Adverse reactions requiring dosage interruption in ≥ 3% of patients who received ZEPZELCA included neutropenia, and hypoalbuminemia.
Dose reductions due to an adverse reaction occurred in 25% of patients who received ZEPZELCA. Adverse reactions requiring dosage reductions in ≥ 3% of patients who received ZEPZELCA included neutropenia, febrile neutropenia and fatigue.
The most common adverse reactions, including laboratory abnormalities, (≥ 20%) were leukopenia, lymphopenia, fatigue, anemia, neutropenia, increased creatinine, increased alanine aminotransferase, increased glucose, thrombocytopenia, nausea, decreased appetite, musculoskeletal pain, decreased albumin, constipation, dyspnea, decreased sodium, increased aspartate aminotransferase, vomiting, cough, decreased magnesium and diarrhea.
Table 3 summarizes the adverse reactions in the SCLC cohort of Study B-005.
| a Graded per NCI CTCAE 4.0. | ||
| b No grade 5 adverse reactions were reported. | ||
| c Includes abdominal pain, abdominal pain upper and abdominal discomfort. | ||
| d Includes musculoskeletal pain, back pain, arthralgia, pain in extremity, musculoskeletal chest pain, neck pain, bone pain and myalgia. | ||
| e Includes cough and productive cough. | ||
| f Includes upper respiratory tract infection, viral upper respiratory tract infection, respiratory tract infection and bronchitis. | ||
| g Includes pneumonia and lung infection. | ||
| h Includes neuropathy peripheral, neuralgia, paresthesia, peripheral sensory neuropathy, hypoesthesia, and hyperesthesia. | ||
|
Adverse Reaction |
ZEPZELCA (n=105) |
|
|
All Gradesa,b (%) |
Grades 3-4 (%) |
|
|
General disorders | ||
|
Fatigue |
77 |
12 |
|
Pyrexia |
13 |
0 |
|
Chest pain |
10 |
0 |
|
Gastrointestinal disorders | ||
|
Nausea |
37 |
0 |
|
Constipation |
31 |
0 |
|
Vomiting |
22 |
0 |
|
Diarrhea |
20 |
4 |
|
Abdominal painc |
11 |
1 |
|
Musculoskeletal and connective tissue disorders | ||
|
Musculoskeletal paind |
33 |
4 |
|
Metabolism and nutrition disorders | ||
|
Decreased appetite |
33 |
1 |
|
Respiratory, thoracic and mediastinal disorders | ||
|
Dyspnea |
31 |
6 |
|
Coughe |
20 |
0 |
|
Infections and infestations | ||
|
Respiratory tract infectionf |
18 |
5 |
|
Pneumoniag |
10 |
7 |
|
Nervous system disorders | ||
|
Peripheral neuropathyh |
11 |
1 |
|
Headache |
10 |
1 |
Clinically relevant adverse reactions in < 10% of patients who received ZEPZELCA include dysgeusia, febrile neutropenia and pneumonitis.
Table 4 summarizes the laboratory abnormalities in Study B-005.
| a The denominator used to calculate the rate varied from 95 to 105 based on the number of patients with a baseline value and at least one post-treatment value. | ||
| b Graded per NCI CTCAE 4.0. | ||
|
Laboratory Abnormality |
ZEPZELCAa (n=105) |
|
|
All Gradesb (%) |
Grades 3-4 (%) |
|
|
Hematology | ||
|
Decreased leukocytes |
79 |
29 |
|
Decreased lymphocytes |
79 |
43 |
|
Decreased hemoglobin |
74 |
10 |
|
Decreased neutrophils |
71 |
46 |
|
Decreased platelets |
37 |
7 |
|
Chemistry | ||
|
Increased creatinine |
69 |
0 |
|
Increased alanine aminotransferase |
66 |
4 |
|
Increased glucose |
52 |
5 |
|
Decreased albumin |
32 |
1 |
|
Decreased sodium |
31 |
7 |
|
Increased aspartate aminotransferase |
26 |
2 |
|
Decreased magnesium |
22 |
0 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ZEPZELCA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General disorders and administration site conditions: Extravasation including tissue necrosis requiring debridement.
Musculoskeletal and Connective Tissue Disorders: Rhabdomyolysis.
Metabolism and nutrition disorders: Tumor lysis syndrome.
Related/similar drugs
7. Drug Interactions
7.1 Effect of Other Drugs on ZEPZELCA
Strong and Moderate CYP3A Inhibitors
Coadministration of ZEPZELCA with a strong or a moderate CYP3A inhibitor increases lurbinectedin systemic exposure [see Clinical Pharmacology (12.3)], which may increase the incidence and severity of adverse reactions to ZEPZELCA.
Avoid grapefruit and Seville oranges during ZEPZELCA treatment, as these contain strong or moderate inhibitors of CYP3A.
Avoid coadministration of ZEPZELCA with strong or moderate CYP3A inhibitors. If coadministration cannot be avoided, reduce the dose of ZEPZELCA [see Dosage and Administration (2.3)].
Strong CYP3A Inducers
Avoid coadministration of ZEPZELCA with strong CYP3A inducers. Coadministration of ZEPZELCA with a strong CYP3A inducer may decrease lurbinectedin systemic exposure, which may decrease the efficacy of ZEPZELCA [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on animal data and its mechanism of action [see Clinical Pharmacology (12.1)], ZEPZELCA can cause fetal harm when administered to a pregnant woman. There are no available data to inform the risk of ZEPZELCA use in pregnant women. Intravenous administration of a single lurbinectedin dose (approximately 0.2 times the 3.2 mg/m2 clinical dose) to pregnant rats during the period of organogenesis caused embryolethality (see Data).
Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In a reproductive toxicity study, administration of a single lurbinectedin dose of 0.6 mg/m2 (approximately 0.2 times of the human dose of 3.2 mg/m2) to pregnant rats on Gestation Day 10 resulted in 100% post-implantation loss.
8.2 Lactation
Risk Summary
There are no data on the presence of lurbinectedin in human milk or its effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions from ZEPZELCA in breastfed children, advise women not to breastfeed during treatment with ZEPZELCA and for 2 weeks after the last dose.
8.3 Females and Males of Reproductive Potential
ZEPZELCA can cause embryolethality at doses lower than the human dose of 3.2 mg/m2 [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating ZEPZELCA.
Contraception
Females
Advise female patients of reproductive potential to use effective contraception during treatment with ZEPZELCA and for 6 months after the last dose.
Males
Advise males with a female sexual partner of reproductive potential to use effective contraception during treatment with ZEPZELCA and for 4 months after the last dose.
8.4 Pediatric Use
The safety and effectiveness of ZEPZELCA in pediatric patients have not been established.
8.5 Geriatric Use
Of the 105 patients with SCLC administered ZEPZELCA in clinical studies, 37 (35%) patients were 65 years of age and older, while 9 (9%) patients were 75 years of age and older. No overall difference in effectiveness was observed between patients aged 65 and older and younger patients.
There was a higher incidence of serious adverse reactions in patients ≥ 65 years of age than in patients < 65 years of age (49% vs. 26%, respectively). The serious adverse reactions most frequently reported in patients ≥ 65 years of age were related to myelosuppression and consisted of febrile neutropenia (11%), neutropenia (11%), thrombocytopenia (8%), and anemia (8%) [see Adverse Reactions (6.1)].
8.6 Hepatic Impairment
Avoid administration of ZEPZELCA in patients with severe hepatic impairment (total bilirubin > 3 × ULN). If administration of ZEPZELCA cannot be avoided, reduce the dose [see Dosage and Administration (2.4)]. Monitor for increased adverse reactions in patients with severe hepatic impairment.
Reduce the dose of ZEPZELCA in patients with moderate hepatic impairment (total bilirubin > 1.5 to 3 × ULN and any AST) [see Dosage and Administration (2.4)]. Monitor for increased adverse reactions in patients with moderate hepatic impairment.
No dose adjustment of ZEPZELCA is recommended for patients with mild hepatic impairment (total bilirubin ≤ ULN and AST > ULN, or total bilirubin 1 to ≤ 1.5 × ULN and any AST) [see Clinical Pharmacology (12.3)].
11. Zepzelca Description
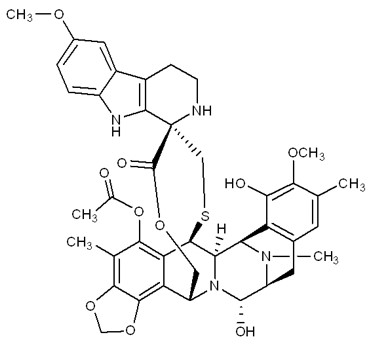
ZEPZELCA is an alkylating drug. The chemical name of ZEPZELCA (lurbinectedin) is (1’R,6R,6aR,7R,13S,14S,16R)-8,14-dihydroxy-6’,9-dimethoxy-4,10,23-trimethyl-19-oxo-2’,3’,4’,6,7,9’,12,13,14,16-decahydro-6aH-spiro[7,13-azano-6,16-(epithiopropanooxymethano) [1,3]dioxolo[7,8]isoquinolino[3,2-b][3]benzazocine-20,1’-pyrido[3,4-b]indol]-5-yl acetate.
The molecular formula is C41H44N4O10S. The molecular weight is 784.87g/mol, and the chemical structure is:
ZEPZELCA for injection 4 mg is supplied as a lyophilized powder in a single-dose vial for reconstitution for intravenous use. The ZEPZELCA lyophilized formulation is comprised of 4 mg lurbinectedin, sucrose (800 mg), lactic acid (22.1 mg), and sodium hydroxide (5.1 mg). Before use, the lyophilizate is reconstituted by addition of 8 mL Sterile Water for Injection USP, yielding a solution containing 0.5 mg/mL lurbinectedin (the calculated concentration is 0.47 mg/mL based on the final volume of 8.5 mL).
12. Zepzelca - Clinical Pharmacology
12.1 Mechanism of Action
Lurbinectedin is an alkylating drug that binds guanine residues in the minor groove of DNA, forming adducts and resulting in a bending of the DNA helix towards the major groove. Adduct formation triggers a cascade of events that can affect the subsequent activity of DNA binding proteins, including some transcription factors, and DNA repair pathways, resulting in perturbation of the cell cycle and eventual cell death.
Lurbinectedin inhibited human monocyte activity in vitro and reduced macrophage infiltration in implanted tumors in mice.
12.2 Pharmacodynamics
Lurbinectedin exposure-response relationships and the pharmacodynamic time-course for efficacy have not been fully characterized.
Increased incidence of Grade 4 neutropenia and Grade ≥ 3 thrombocytopenia were observed with increased lurbinectedin exposure.
Cardiac Electrophysiology
No large mean increase in QTc (i.e., > 20 ms) was detected at the recommended dose of 3.2 mg/m2.
12.3 Pharmacokinetics
Following the approved recommended dosage, geometric mean (%CV) of plasma Cmax and AUC0-inf, were 107 µg/L (79%) and 551 µg•h/L (94%), respectively. No accumulation of lurbinectedin in plasma is observed upon administrations every 3 weeks.
Distribution
The volume of distribution of lurbinectedin at steady state is 504 L (39%). Plasma protein binding is approximately 99%, to both albumin and α-1-acid glycoprotein.
Elimination
The terminal half-life of lurbinectedin is 51 hours. Total plasma clearance of lurbinectedin is 11 L/h (50%).
Metabolism
Lurbinectedin is metabolized by CYP3A in vitro.
Excretion
After a single dose of radiolabeled lurbinectedin, 89% of the radioactivity was recovered in feces (< 0.2% unchanged) and 6% in urine (1% unchanged).
Specific Populations
No clinically significant differences in the pharmacokinetics of lurbinectedin were identified based on age (18-85 years), sex, body weight (39-154 kg), or mild to moderate renal impairment (CLcr 30 to 89 mL/min). The effects of severe renal impairment (CLcr < 30 mL/min) on the pharmacokinetics of lurbinectedin have not been studied.
Hepatic Impairment
No clinically significant differences in the pharmacokinetics of lurbinectedin were identified for patients with mild hepatic impairment (total bilirubin ≤ ULN and AST > ULN or total bilirubin 1 to 1.5 × ULN and any AST) compared to that of patients with normal hepatic function.
No clinically significant differences in the pharmacokinetics of lurbinectedin were identified for patients with moderate hepatic impairment (total bilirubin > 1.5 to 3 × ULN and any AST) who received a lurbinectedin dose of 1.6 mg/m2 compared to that of patients with mild hepatic impairment who received a dose of 3.2 mg/m2.
No clinically significant differences in the pharmacokinetics of lurbinectedin were identified for patients with severe hepatic impairment (total bilirubin > 3 × ULN) who received a lurbinectedin dose of 1.6 mg/m2 compared to that of patients with mild hepatic impairment who received a dose of 3.2 mg/m2.
Drug Interactions Studies
Clinical Studies and Model-Informed Approaches
Effects of CYP3A Inhibitors on Lurbinectedin
Strong CYP3A inhibitors: Coadministration of itraconazole (200 mg once daily) increased the systemic exposure (AUC) of total lurbinectedin by 2.7-fold and unbound lurbinectedin by 2.4‑fold.
Moderate CYP3A inhibitors: Coadministration of verapamil (80 mg every 8 hours) and erythromycin (500 mg every 6 hours) is predicted to increase lurbinectedin AUC by 2.3-fold and 2.1-fold, respectively.
Weak CYP3A inhibitors: Coadministration of fluvoxamine (150 mg every 12 hours) is predicted to increase lurbinectedin AUC by 1.3-fold.
Effects of CYP3A Inducers on Lurbinectedin
Coadministration of bosentan (a moderate CYP3A inducer) decreased systemic exposure (AUC) of total lurbinectedin by 20% and unbound lurbinectedin by 19%. These changes are not considered clinically relevant.
In vitro Studies
Cytochrome P450 (CYP) Enzymes: Lurbinectedin is not an inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4.
Lurbinectedin is not an inducer of CYP1A2 or CYP3A4.
Transporter Systems: Lurbinectedin is a substrate of MDR1, but is not a substrate of OATB1P1, OATP1B3, OCT1, or MATE1.
Lurbinectedin inhibits MDR1, OATP1B1, OATP1B3, and OCT1 but not BCRP, BSEP, MATE1, OAT1, OAT3, or OCT2.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity testing of lurbinectedin has not been performed. Lurbinectedin is genotoxic to mammalian cells in the presence and absence of metabolic activation. Lurbinectedin was not mutagenic in vitro in a bacterial reverse mutation (Ames) assay.
Fertility studies with lurbinectedin were not performed. There were no findings in reproductive organs in general toxicology studies in rats, dogs, or monkeys; however, the highest doses and exposures in these studies were all at levels lower than those at the human dose of 3.2 mg/m2.
14. Clinical Studies
PM1183-B-005-14 (Study B-005; NCT02454972) is a multicenter, open-label, multi-cohort trial evaluating ZEPZELCA as a single agent in patients with advanced or metastatic solid tumors. A cohort of patients with small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy received ZEPZELCA 3.2 mg/m2 by intravenous infusion every 21 days (one cycle). Patients received a median of 4 cycles of ZEPZELCA (range 1 to 24 cycles). The trial excluded patients with central nervous system (CNS) involvement, grade ≥ 3 dyspnea, daily intermittent oxygen requirement, hepatitis or cirrhosis, and immunocompromised patients. Tumor assessments were conducted every 6 weeks for the first 18 weeks and every 9 weeks thereafter. The major efficacy outcome measure was confirmed investigator-assessed overall response rate (ORR). Additional efficacy outcome measures included duration of response (DoR), and an Independent Review Committee (IRC) assessed ORR using Response Evaluation Criteria in Solid Tumors (RECIST v1.1).
A total of 105 patients with SCLC who progressed on or after platinum-based chemotherapy were enrolled. The median age was 60 years (range: 40 to 83) with 65% of patients < 65 years and 35% of patients ≥ 65 years, and 60% were male. The majority (75%) of the patients were White, 1% were Asian, 1% were Black and 23% were not reported. Baseline ECOG performance status was 0 or 1 in 92% of patients, and 92% were former/current smokers. All patients received at least one line of platinum-based chemotherapy (range 1-2 lines), and prior radiotherapy had been administered to 71% of patients. Eight patients (8%) had prior immunotherapy in addition to platinum-based chemotherapy. Sixty patients (57%) had platinum-sensitive SCLC, defined as recurrence or progression ≥ 90 days after the last dose of platinum-containing therapy (chemotherapy free interval [CTFI] ≥ 90 days). The remaining 45 patients had platinum-resistant SCLC, defined as recurrence or progression < 90 days after the last dose of platinum-containing therapy (CTFI < 90 days).
Table 5 summarizes investigator-assessed and independent review committee assessed key efficacy measures in all patients and in platinum-resistant and platinum-sensitive subgroups.
| CI: confidence interval, CTFI: chemotherapy free interval. | |||
| a Confirmed overall response rate. | |||
| b Based on observed duration of response. | |||
|
Investigator Assessed Responsea |
ZEPZELCA |
ZEPZELCA |
ZEPZELCA |
|
Overall Response Rate (95% CI) |
35% (26%, 45%) |
22% (11%, 37%) |
45% (32%, 58%) |
|
Complete response |
0% |
0% |
0% |
|
Partial response |
35% |
22% |
45% |
|
Duration of Response | |||
|
Median in months (95% CI) |
5.3 (4.1, 6.4) |
4.7 (2.6, 5.6) |
6.2 (3.5, 7.3) |
|
% with ≥ 6 monthsb |
35% |
10% |
44% |
|
Independent Review Committee Assessed Responsea |
All Patients |
CTFI < 90 days |
CTFI ≥ 90 days |
|
Overall Response Rate (95% CI) |
30% (22%, 40%) |
13% (5%, 27%) |
43% (31%, 57%) |
|
Complete response |
0% |
0% |
0% |
|
Partial response |
30% |
13% |
43% |
|
Duration of Response | |||
|
Median in months (95% CI) |
5.1 (4.9, 6.4) |
4.8 (2.4, 5.3) |
5.3 (4.9, 7.0) |
|
% with ≥ 6 monthsb |
25% |
0% |
31% |
16. How is Zepzelca supplied
How Supplied
ZEPZELCA (lurbinectedin) for injection is supplied as a sterile, preservative-free, white to off-white lyophilized powder in a single-dose clear glass vial. Each carton (NDC 68727-712-01) contains 4 mg in one single-dose vial.
Storage and Handling
Store refrigerated at 2°C to 8°C (36°F to 46°F).
ZEPZELCA is a hazardous drug. Follow applicable special handling and disposal procedures1.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Myelosuppression
Advise patients to immediately contact their healthcare provider for fever, other signs of infection, unusual bruising, bleeding, tiredness or pallor [see Warnings and Precautions (5.1)].
Hepatotoxicity
Advise patients to contact their healthcare provider immediately for signs and symptoms suggestive of hepatotoxicity [see Warnings and Precautions (5.2)].
Extravasation Resulting in Tissue Necrosis
Advise patients to contact their healthcare provider immediately for signs and symptoms of extravasation. The time to onset of necrosis after extravasation may vary [see Warnings and Precautions (5.3)].
Rhabdomyolysis
Advise patients to contact their healthcare provider immediately for signs and symptoms of rhabdomyolysis [see Warnings and Precautions (5.4)].
Embryo-Fetal Toxicity
- •
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.5) and Use in Specific Populations (8.1)].
- •
- Advise females of reproductive potential to use effective contraception during treatment with ZEPZELCA and for 6 months after the last dose [see Use in Specific Populations (8.3)].
- •
- Advise males with female partners of reproductive potential to use effective contraception during treatment with ZEPZELCA and for 4 months after the last dose [see Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed during treatment with ZEPZELCA and for at least 2 weeks after the last dose [see Use in Specific Populations (8.2)].
Drug Interactions
Advise patients to inform their healthcare providers of all concomitant medications, herbal and dietary supplements. Advise patients to avoid grapefruit products and Seville oranges during treatment with ZEPZELCA [see Drug Interactions (7.1)].
Distributed by:
Jazz Pharmaceuticals, Inc.
Palo Alto, CA 94306
Under license from Pharma Mar, S.A.
Protected by U.S. Patent No. 7,763,615
Patient Package Insert
|
PATIENT INFORMATION ZEPZELCA® (zep zel' kah) (lurbinectedin) for injection |
|
What is ZEPZELCA?
It is not known if ZEPZELCA is safe and effective in children. |
|
Before receiving ZEPZELCA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Certain other medicines may affect how ZEPZELCA works. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
|
How will I receive ZEPZELCA?
|
|
What should I avoid while using ZEPZELCA?
|
|
What are the possible side effects of ZEPZELCA? ZEPZELCA can cause serious side effects, including:
○ fever or any other signs of infection ○ unusual bruising or bleeding ○ tiredness ○ pale colored skin
○ loss of appetite ○ nausea or vomiting ○ pain on the right side of your stomach-area (abdomen)
Your healthcare provider may temporarily stop treatment, lower your dose, or permanently stop ZEPZELCA if you develop serious side effects during treatment with ZEPZELCA. The most common side effects of ZEPZELCA include:
These are not all of the possible side effects of ZEPZELCA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088. |
|
General information about the safe and effective use of ZEPZELCA. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about ZEPZELCA that is written for health professionals. |
|
What are the ingredients in ZEPZELCA? Distributed by: Jazz Pharmaceuticals, Inc. Palo Alto, CA 94306 Under license from Pharma Mar, S.A. ZEPZELCA is a registered trademark of Pharma Mar, S.A. For more information, go to www.ZEPZELCA.com or call 1-800-520-5568. |
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 7/2025
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 68727-712-01
ZEPZELCA
(lurbinectedin)
for injection
4 mg per vial
FOR INTRAVENOUS INFUSION ONLY
Reconstitute before further dilution.
Each single-dose vial contains 4 mg of lurbinectedin
as a sterile lyophilized powder
Rx Only
Single-dose vial
Discard unused portion.
Caution: Cytotoxic agent
| ZEPZELCA
lurbinectedin injection, powder, lyophilized, for solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Jazz Pharmaceuticals, Inc. (135926363) |
| Registrant - Jazz Pharmaceuticals Ireland Limited (896650210) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Oncology GmbH | 344276063 | MANUFACTURE(68727-712) , PACK(68727-712) , ANALYSIS(68727-712) , LABEL(68727-712) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharma Mar, SA | 464884501 | API MANUFACTURE(68727-712) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AndersonBrecon Inc (Specialty Pharma Center SPC) | 098908572 | PACK(68727-712) , LABEL(68727-712) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GP Pharm SA | 462006581 | MANUFACTURE(68727-712) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Jazz Pharmaceuticals Ireland Limited | 896650210 | MANUFACTURE(68727-712) | |
More about Zepzelca (lurbinectedin)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: alkylating agents
- En español