Niferex: Package Insert / Prescribing Info
Package insert / product label
Generic name: iron supplement
Dosage form: tablet
Drug class: Iron products
Medically reviewed by Drugs.com. Last updated on Jan 13, 2025.
On This Page
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately.
DESCRIPTION: Niferex™ for oral administrationis a prenatal/postnatal iron supplement that is a round, copper colored tablet with "NxFE" embossed on one side.
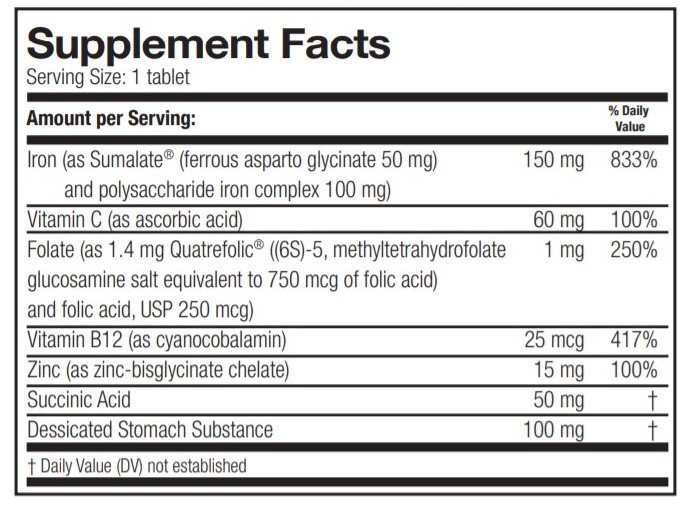
OTHER INGREDIENTS: Croscarmellose sodium, dicalcium phosphate, magnesium stearate, microcrystalline cellulose, silicon dioxide, stearic acid. Coating contains: HPMC, triacetin, titanium dioxide, Candurin® Orange (FD&C Blue #1, FD&C Red #40, FD&C Yellow #6). This product contains FD&C Yellow #6.
INDICATIONS: Niferex™ is a multivitamin/multimineral dietary supplement indicated for use in improving the nutritional status of patients with iron deficiency.
CONTRAINDICATIONS: Niferex™ is contraindicated in patients with a known hypersensitivity to any of the ingredients.
PRECAUTIONS: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. Folic acid in doses above 1.0 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
ADVERSE REACTIONS: Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
HOW SUPPLIED: Bottles of 30 tablets (75854-321-30). The listed product number is not a National Drug Code. Instead, Avion Pharmaceuticals has assigned a product code formatted according to standard industry practice to meet the formatting requirements of pharmacy and healthcare insurance computer systems.
STORAGE: Store at 20° - 25°C (68° - 77°F); excursions permitted to 15° - 30°C (59° - 86°F) [See USP Controlled Room Temperature.]
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Quatrefolic® is a registered trademark of Gnosis, SpA. Covered by one or more claims of U.S. Patent #7,947,662 CAS #1181972-37-1
Sumalate® is a registered trademark of Albion Laboratories, Inc., covered by one or more claims of U.S. Patent Nos. 6,716,814, 8,007,846 and 8,425,956.
MANUFACTURED FOR:
Avion Pharmaceuticals, LLC
Alpharetta, GA 30005 1-888-61-AVION
Rev. 0119-01 AV-428
THESE STATEMENTS HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION. THIS PRODUCT IS NOT INTENDED TO DIAGNOSE, TREAT, CURE OR INTENDED TO DIAGNOSE, PREVENT ANY DISEASE.
| NIFEREX
ferrous asparto glycinate, iron, ascorbic acid, folic acid, cyanocobalamin, zinc, succinic acid, and intrinsic factor tablet |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Labeler - Avion Pharmaceuticals, LLC (040348516) |
| Registrant - Avion Pharmaceuticals, LLC (965450542) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Avion Pharmaceuticals, LLC | 040348516 | manufacture(75854-321) | |
Related/similar drugs
More about iron polysaccharide
- Check interactions
- Compare alternatives
- Reviews (3)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: iron products
- En español
Patient resources
- Iron polysaccharide drug information
- Polysaccharide-Iron Complex Capsules and Tablets
- Polysaccharide-Iron Complex Liquid