Naftifine Cream: Package Insert / Prescribing Info
Package insert / product label
Generic name: naftifine hydrochloride
Dosage form: cream
Drug class: Topical antifungals
Medically reviewed by Drugs.com. Last updated on Jun 2, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
NAFTIFINE HYDROCHLORIDE cream, for topical use
Initial U.S. Approval: 1988
Indications and Usage for Naftifine Cream
Naftifine Hydrochloride Cream is an allylamine antifungal indicated for the treatment of interdigital tinea pedis, tinea cruris, and tinea corporis caused by the organism Trichophyton rubrum. (1)
Naftifine Cream Dosage and Administration
Dosage Forms and Strengths
Cream: 2% (3)
Contraindications
None (4)
Warnings and Precautions
Discontinue treatment if redness or irritation develops with Naftifine Hydrochloride Cream use. ( 5.1)
Adverse Reactions/Side Effects
The most common adverse reaction (≥1%) is pruritus. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Taro Pharmaceuticals U.S.A., Inc., at 1-866-923-4914 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2018
Full Prescribing Information
1. Indications and Usage for Naftifine Cream
Naftifine Hydrochloride Cream is indicated for the treatment of interdigital tinea pedis, tinea cruris, and tinea corporis caused by the organism Trichophyton rubrum.
2. Naftifine Cream Dosage and Administration
For topical use only. Naftifine Hydrochloride Cream is not for ophthalmic, oral, or intravaginal use. Apply a thin layer of Naftifine Hydrochloride Cream once-daily to the affected areas plus a ½ inch margin of healthy surrounding skin for 2 weeks.
3. Dosage Forms and Strengths
Each gram of Naftifine Hydrochloride Cream contains 20 mg of naftifine hydrochloride (2%) in a white to off-white base.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
During clinical trials, 903 subjects were exposed to naftifine 1% and 2% cream formulations. A total of 564 subjects with interdigital tinea pedis, tinea cruris, or tinea corporis were treated with Naftifine Hydrochloride Cream.
In two randomized, vehicle-controlled trials (400 subjects were treated with Naftifine Hydrochloride Cream). The population was 12 to 88 years old, primarily male (79%), 48% Caucasian, 36% Black or African American, 40% Hispanic or Latino and had either predominantly interdigital tinea pedis or tinea cruris. Most subjects received doses once-daily, topically, for 2 weeks to cover the affected skin areas plus a ½ inch margin of surrounding healthy skin. In the two vehicle-controlled trials, 17.5% of Naftifine Hydrochloride Cream treated subjects experienced an adverse reaction compared with 19.3% of vehicle subjects. The most common adverse reaction (≥1%) is pruritus. Most adverse reactions were mild in severity. The incidence of adverse reactions in the Naftifine Hydrochloride Cream treated population was not significantly different than in the vehicle treated population.
In a third randomized, vehicle-controlled trial, 116 pediatric subjects with tinea corporis were treated with Naftifine Hydrochloride Cream. The population was aged ≥2 to <18 years (mean age of 9 years), predominately male (61%), 47% White, 51% Black or African American, 92% Hispanic or Latino, and infected with tinea corporis. Naftifine Hydrochloride Cream was topically applied once daily for 2 weeks to all affected body surface areas with tinea corporis plus a ½ inch margin of healthy skin surrounding the affected lesions. The incidence of adverse reactions in the Naftifine Hydrochloride Cream treated population was not significantly different than in the vehicle treated population.
In two open-label pediatric pharmacokinetics and safety trials, 49 pediatric subjects 2 to <18 years of age with interdigital tinea pedis, tinea cruris, and tinea corporis received Naftifine Hydrochloride Cream. The incidence of adverse reactions in the pediatric population was similar to that observed in the adult population.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of naftifine hydrochloride: redness/irritation, inflammation, maceration, swelling, burning, blisters, serous drainage, crusting, headache, dizziness, leukopenia, agranulocytosis.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Related/similar drugs
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available data with Naftifine Hydrochloride Cream in pregnant women to inform the drug-associated risk for major birth defects and miscarriage. In animal reproduction studies, no adverse effects on embryofetal development were seen at oral doses administered during the period of organogenesis up to 18 times the maximum recommended human dose (MRHD) in pregnant rats or subcutaneous doses administered during the period of organogenesis up to 2 times the MRHD in pregnant rats or 4 times the MRHD in pregnant rabbits [see Data] .
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Systemic embryofetal development studies were conducted in rats and rabbits. For the comparison of animal to human doses based on body surface area comparison (mg/m 2), the MRHD is set at 8 g 2% cream per day (2.67 mg/kg/day for a 60 kg individual).
Oral doses of 30 mg/kg/day, 100 mg/kg/day and 300 mg/kg/day naftifine hydrochloride were administered during the period of organogenesis to pregnant female rats. No treatment-related effects on embryofetal development were noted at doses up to 300 mg/kg/day (18 times MRHD). Subcutaneous doses of 10 mg/kg/day and 30 mg/kg/day naftifine hydrochloride were administered during the period of organogenesis to pregnant female rats. No treatment-related effects on embryofetal development were noted at 30 mg/kg/day (2 times MRHD). Subcutaneous doses of 3 mg/kg/day, 10 mg/kg/day and 30 mg/kg/day naftifine hydrochloride were administered during the period of organogenesis to pregnant female rabbits. No treatment-related effects on embryofetal development were noted at 30 mg/kg/day (4 times MRHD).
A peri- and post-natal development study was conducted in rats. Oral doses of 30 mg/kg/day, 100 mg/kg/day and 300 mg/kg/day naftifine hydrochloride were administered to female rats from gestational day 14 to lactation day 21. Reduced body weight gain of females during gestation and of the offspring during lactation was noted at 300 mg/kg/day (18 times MRHD). No developmental toxicity was noted at 100 mg/kg/day (6 times MRHD).
8.2 Lactation
Risk Summary
There is no information available on the presence of Naftifine Hydrochloride Cream in human milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. The lack of clinical data during lactation precludes a clear determination of the risk of Naftifine Hydrochloride Cream to an infant during lactation; therefore, the development and health benefits of breastfeeding should be considered along with the mother's clinical need for Naftifine Hydrochloride Cream and any potential adverse effects on the breastfed infant from Naftifine Hydrochloride Cream or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of Naftifine Hydrochloride Cream have been established in pediatric patients age 12 and above with interdigital tinea pedis and tinea cruris and age 2 and above with tinea corporis [see Clinical Studies (14)and Clinical Pharmacology (12.3)] .
Use of Naftifine Hydrochloride Cream in these age groups is supported by evidence from adequate and well controlled studies in adults and children, with additional safety and PK data from two open label trials conducted in 49 pediatric subjects exposed to Naftifine Hydrochloride Cream [see Clinical Studies (14)and Clinical Pharmacology (12.3)] .
Safety and effectiveness of Naftifine Hydrochloride Cream in the treatment of tinea cruris and interdigital tinea pedis in pediatric patients less than 12 years of age have not been established. Safety and effectiveness of Naftifine Hydrochloride Cream in the treatment of tinea corporis in pediatric patients less than 2 years of age have not been established.
11. Naftifine Cream Description
Naftifine Hydrochloride Cream is a white to off-white cream for topical use only. Each gram of Naftifine Hydrochloride Cream contains 20 mg of naftifine hydrochloride (2%), a synthetic allylamine antifungal compound.
Chemically, naftifine HCl is (E)-N-Cinnamyl-N-methyl-1-napthalenemethylamine hydrochloride.
The molecular formula is C 21H 21N∙HCl with a molecular weight of 323.86.
The structural formula of naftifine hydrochloride is:
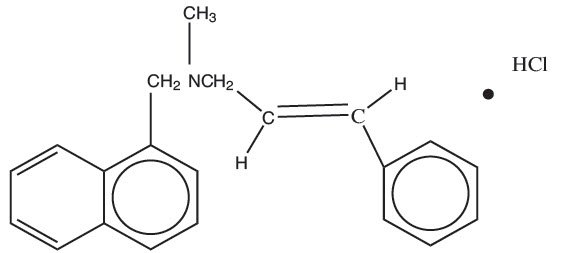
Naftifine Hydrochloride Cream contains the following inactive ingredients: benzyl alcohol, cetyl alcohol, cetyl esters wax, hydrochloric acid, isopropyl myristate, polysorbate 60, purified water, sodium hydroxide, sorbitan monostearate, and stearyl alcohol.
12. Naftifine Cream - Clinical Pharmacology
12.1 Mechanism of Action
Naftifine Hydrochloride Cream is a topical antifungal drug [see Clinical Pharmacology (12.4)]
12.2 Pharmacodynamics
The pharmacodynamics of Naftifine Hydrochloride Cream have not been established.
12.3 Pharmacokinetics
In vitroand in vivobioavailability studies have demonstrated that naftifine penetrates the stratum corneum in sufficient concentration to inhibit the growth of dermatophytes.
The pharmacokinetics of Naftifine Hydrochloride Cream was evaluated following once-daily topical application for 2 weeks to 21 adult subjects, both males and females, with both tinea pedis and tinea cruris. The median total amount of cream applied was 6.4 g (range 5.3 to 7.5 g) per day. The results showed that the systemic exposure (i.e., maximum concentration (C max) and area under the curve from time 0 to 24 hours (AUC 0-24)) to naftifine increased over the 2 week treatment period in all the 21 subjects. Geometric mean (coefficient of variation or CV%) AUC 0-24was 117 (41.2) ng*hr/mL on Day 1, and 204 (28.5) ng*hr/mL on Day 14. Geometric mean (CV%) C maxwas 7 ng/mL (55.6) on Day 1 and 11 ng/mL (29.3) on Day 14. Median time to C max(T max) was 8 hours (range: 4 to 24 hours) on Day 1 and 6 hours (range: 0 to 16 hours) on Day 14. Accumulation after 14 days of topical application was less than two fold. Trough concentrations generally increased throughout the 14 day study period. Naftifine continued to be detected in plasma in 13/21 (62%) subjects on Day 28, the mean (standard deviation or SD) plasma concentrations were 1.6 ± 0.5 ng/mL (range below limit of quantitation (BLQ) to 3 ng/mL). In the same pharmacokinetic trial conducted in patients with tinea pedis and tinea cruris, median fraction of the dose excreted in urine during the treatment period was 0.0016% on Day 1 versus 0.0020% on Day 14.
In a second trial that enrolled 22 subjects, the pharmacokinetics of Naftifine Hydrochloride Cream was evaluated in 20 pediatric subjects 13 to <18 years of age with both tinea pedis and tinea cruris. Subjects were treated with a median dose of 8.1 g (range 6.6 to 10.1 g) applied to the affected areas once daily for 2 weeks. The results showed that the systemic exposure increased over the treatment period. Geometric mean (CV%) AUC 0-24was 138 (50.2) ng*hr/mL on Day 1, and 192 (74.9) ng*hr/mL on Day 14. Geometric mean (CV%) C maxwas 9.21 ng/mL (48.4) on Day 1 and 12.7 ng/mL (67.2) on Day 14. Median fraction of the dose excreted in urine during the treatment period was 0.0030% on Day 1 and 0.0033% on Day 14.
A third trial evaluated the pharmacokinetics of Naftifine Hydrochloride Cream in 27 pediatric subjects 2 to < 12 years of age with at least moderate tinea corporis. Subjects were divided into younger (ages 2 to < 6 years, 17 subjects) and older (6 to <12 years, 10 subjects) groups. Median doses of 1.3 g (range 1 to 3.1 g) and 2.3 g (range 2.2 to 4.2 g) were applied once-daily for 2 weeks in the younger and older groups, respectively, to the affected area plus a ½ inch margin. Plasma and urine pharmacokinetic assessments were conducted on Day 1 in the older group only and on Day 14 in both groups. All subjects showed measurable levels of naftifine in plasma after topical application of Naftifine Hydrochloride Cream. Following a single dose on Day 1 in subjects 6 to < 12 years of age, the geometric mean (CV%) values of C maxand AUC 0-24were 3.60 (76.6) ng/mL and 49.8 (64.4) ng*h/mL, respectively. On Day 14 in this group, the C maxand AUC 0-24were 3.31 (51.2) ng/mL and 52.4 (49.2) ng*h/mL, respectively. In subjects 2 to < 6 years of age on Day 14, the C maxand AUC 0-24were 3.98 (186) ng/mL and 54.8 (150) ng*h/mL, respectively. In the older group of subjects 6 to 12 years of age, the systemic exposures (both C maxand AUC 0-24) on Days 1 and 14 were comparable. The median fraction of the dose excreted into urine over 24 hours following drug applications on Day 1 and Day 14 was 0.0029% and 0.0014%, respectively.
12.4 Microbiology
Although the exact mechanism of action against fungi is not known, naftifine hydrochloride appears to interfere with sterol biosynthesis by inhibiting the enzyme squalene 2, 3-epoxidase.This inhibition of enzyme activity results in decreased amounts of sterols, especially ergosterol, and a corresponding accumulation of squalene in the cells.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year dermal carcinogenicity study, naftifine hydrochloride cream was administered to Sprague-Dawley rats at topical doses of 1%, 2% and 3% (10 mg/kg/day, 20 mg/kg/day, and 30 mg/kg/day naftifine hydrochloride). No drug-related tumors were noted in this study up to the highest dose evaluated in this study of 30 mg/kg/day (12 times MRHD based on AUC comparison).
Naftifine hydrochloride revealed no evidence of mutagenic or clastogenic potential based on the results of two in vitrogenotoxicity tests (Ames assay and Chinese hamster ovary cell chromosome aberration assay) and one in vivogenotoxicity test (mouse bone marrow micronucleus assay).
Oral administration of naftifine hydrochloride to rats, throughout mating, gestation, parturition and lactation, demonstrated no effects on growth, fertility or reproduction, at doses up to 100 mg/kg/day (6 times MRHD based on mg/m 2comparison).
14. Clinical Studies
14.1 Tinea Cruris
Naftifine Hydrochloride Cream has been investigated for safety and efficacy in a randomized, double-blind, vehicle-controlled, multi-center trial in 146 subjects with symptomatic and dermatophyte culture positive tinea cruris. Subjects were randomized to receive Naftifine Hydrochloride Cream or vehicle. Subjects applied Naftifine Hydrochloride Cream or vehicle to the affected area plus a ½-inch margin of healthy skin surrounding the affected area once-daily for 2 weeks. Signs and symptoms of tinea cruris (presence or absence of erythema, pruritus, and scaling) were assessed, and KOH examination and dermatophyte culture were performed at the primary efficacy endpoint at week 4.
The mean age of the trial population was 47 years and 87% were male and 43% were white. At baseline, subjects were confirmed to have signs and symptoms of tinea cruris, positive KOH exam, and confirmed dermatophyte presence based on culture results from a central mycology laboratory. The analysis of the intent-to-treat population was a comparison of the proportions of subjects with a complete cure at the week 4 visit (see Table 1). Complete cure was defined as both clinical cure (absence of erythema, pruritus, and scaling) and mycological cure (negative KOH and dermatophyte culture).
The percentage of subjects experiencing clinical cure and the percentage of subjects experiencing mycological cure at week 4 are presented individually in Table 1 below.
| Endpoint | Naftifine Hydrochloride Cream, 2%
N=75 | Vehicle
N=71 |
|---|---|---|
|
||
| Complete Cure * | 19 (25%) | 2 (3%) |
| Effective Treatment † | 45 (60%) | 7 (10%) |
| Mycological Cure ‡ | 54 (72%) | 11 (16%) |
14.2 Interdigital Tinea Pedis
Naftifine Hydrochloride Cream has been investigated for efficacy in a randomized, double-blind, vehicle-controlled, multi-center trial in 217 subjects with symptomatic and dermatophyte culture positive interdigital tinea pedis. Subjects were randomized to receive Naftifine Hydrochloride Cream or vehicle. Subjects applied Naftifine Hydrochloride Cream or vehicle to the affected area of the foot plus a ½-inch margin of healthy skin surrounding the affected area once-daily for 2 weeks. Signs and symptoms of interdigital tinea pedis (presence or absence of erythema, pruritus, and scaling) were assessed and KOH examination and dermatophyte culture was performed at the primary efficacy endpoint at week 6.
The mean age of the trial population was 42 years and 71% were male and 57% were white. At baseline, subjects were confirmed to have signs and symptoms of interdigital tinea pedis, positive KOH exam, and confirmed dermatophyte culture. The primary efficacy endpoint was the proportions of subjects with a complete cure at the week 6 visit (see Table 2). Complete cure was defined as both a clinical cure (absence of erythema, pruritis, and scaling) and mycological cure (negative KOH and dermatophyte culture).
The efficacy results at week 6, four weeks following the end of treatment, are presented in Table 2 below. Naftifine Hydrochloride Cream demonstrated complete cure in subjects with interdigital tinea pedis, but complete cure in subjects with only moccasin type tinea pedis was not demonstrated.
| Endpoint | Naftifine Hydrochloride Cream, 2%
N=147 | Vehicle
N=70 |
|---|---|---|
|
||
| Complete Cure * | 26 (18%) | 5 (7%) |
| Effective Treatment † | 83 (57%) | 14 (20%) |
| Mycological Cure ‡ | 99 (67%) | 15 (21%) |
14.3 Tinea Corporis
Naftifine Hydochloride Cream has been investigated for safety and efficacy in a randomized, double-blind, vehicle-controlled, multi-center trial in 184 subjects with symptomatic and dermatophyte culture positive tinea corporis. Subjects were randomized to receive Naftifine Hydrochloride Cream or vehicle. Subjects applied the study agent to all affected body surface areas with tinea corporis plus a ½ inch margin of healthy skin surrounding the affected lesions for two weeks. Signs and symptoms of tinea corporis (presence or absence of erythema, induration, and pruritus) were assessed and KOH examination and dermatophyte culture were performed for the assessment of primary efficacy endpoint at Day 21.
The trial population was pediatric (≥2 to <18 years of age) with a median age of 9 years (Naftifine Hydrochloride Cream) or 8 years (vehicle); 61% of subjects were male and 45% were white. At baseline, subjects were confirmed to have signs and symptoms of tinea corporis, positive KOH exam, and confirmed dermatophyte culture. The primary efficacy endpoint was the proportions of subjects with a complete cure at the Day 21 visit. Complete cure was defined as both a clinical cure (absence of erythema, induration, and pruritus on all lesions present at baseline) and mycological cure (negative KOH and dermatophyte culture).
The efficacy results at Day 21, one week following the end of treatment, are presented in Table 3 below.
| Endpoint | Naftifine Hydrochloride Cream, 2%
N=91 | Vehicle
N=93 |
|---|---|---|
|
||
| Complete Cure * | 42 (46%) | 26 (28%) |
| Effective Treatment † | 53 (58%) | 32 (34%) |
| Mycological Cure ‡ | 57 (63%) | 36 (39%) |
16. How is Naftifine Cream supplied
17. Patient Counseling Information
- Inform patients that Naftifine Hydrochloride Cream is for topical use only. Naftifine Hydrochloride Cream is not intended for oral, intravaginal or ophthalmic use.
- If irritation or sensitivity develops with the use of Naftifine Hydrochloride Cream treatment should be discontinued and appropriate therapy instituted. Patients should be directed to contact their physician if these conditions develop following use of Naftifine Hydrochloride Cream.
| NAFTIFINE HYDROCHLORIDE
naftifine hydrochloride cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Sun Pharmaceutical Industries, Inc. (146974886) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sun Pharma Canada Inc. | 243339023 | manufacture(51672-1368) | |
More about naftifine topical
- Compare alternatives
- Pricing & coupons
- Reviews (6)
- Side effects
- Dosage information
- During pregnancy
- Drug class: topical antifungals
- Breastfeeding
- En español

