FerraPlus 90: Package Insert / Prescribing Info
Package insert / product label
Generic name: docusate sodium, folic acid, iron, cyanocobalamin, and ascorbic acid
Dosage form: tablet, film coated
Drug classes: Iron products, Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Feb 27, 2025.
On This Page
CONTRAINDICATIONS
Hypersensitivity to any of the ingredients. Hemolytic anemia, hemochromatosis, and hemosiderosis are contraindications to iron therapy.
Warnings
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient.

Precautions
General: Take 2 hours after meals. Do not exceed recommended dose. Discontinue use if symptoms of intolerance appear. The type of anemia and underlying cause or causes should be determined before starting therapy with FerraPlus 90 tablets. Ensure Hgb, Hct, reticulocyte count are determined before starting therapy and periodically thereafter during prolonged treatment. Periodically review therapy to determine if it needs to be continued without change or if a dose change or if a dose change is indicated.
Folic Acid: Folic acid in doses above 0.1 mg daily may obscure pernicious anemia assessment, such that hematologic remission can occur while neurological manifestations remain progressive. Pernicious anemia should be excluded before using these products since folic acid may mask the symptoms of pernicious anemia.
Pediatric Use: Safety and effectiveness of this product have not been established in pediatric patients.
Geriatric Use: Safety and effectiveness of this product have not been established in elderly patients.
DRUG INTERACTIONS
Prescriber should be aware of a number of iron/drug interactions, including antacids, tetracyclines, or fluoroquinolones.
ADVERSE REACTIONS
Adverse reactions with iron therapy may include GI irritation, constipation, diarrhea, nausea, vomiting, and dark stools. Adverse reactions with iron therapy are usually transient. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
Related/similar drugs
OVERDOSAGE
Symptoms: Abdominal pain, metabolic acidosis, anuria, CNS damage, coma, convulsions, death, dehydration, diffuse vascular congestion, hepatic cirrhosis, hypotension, hypothermia, lethargy, nausea, vomiting, diarrhea, tarry stools, melena, hematemesis, tachycardia, hyperglycemia, drowsiness, pallor, cyanosis, lassitude, seizures, and shock.
FerraPlus 90 Dosage and Administration
One tablet daily or as directed by a physician. Do not chew tablet.
STORAGE
Store at 20° - 25°C (68° - 77°F), excursions permitted to 15° - 30°C (59° - 86°F) [See USP Controlled Room Temperature]. Protect from moisture (contact with moisture can discolor or erode the tablet). Dispense in a tight container with a child-resistant closure.
KEEP OUT OF THE REACH OF CHILDREN.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories at 1-877-482-3788 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Customer Service: 1-888-987-4436 Rev. 05/2020
Manufactured for: 
Trigen Laboratories, LLC
Bridgewater, NJ 08807
| FERRAPLUS 90
docusate sodium, folic acid, carbonyl iron, cyanocobalamin, and ascorbic acid tablet, film coated |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
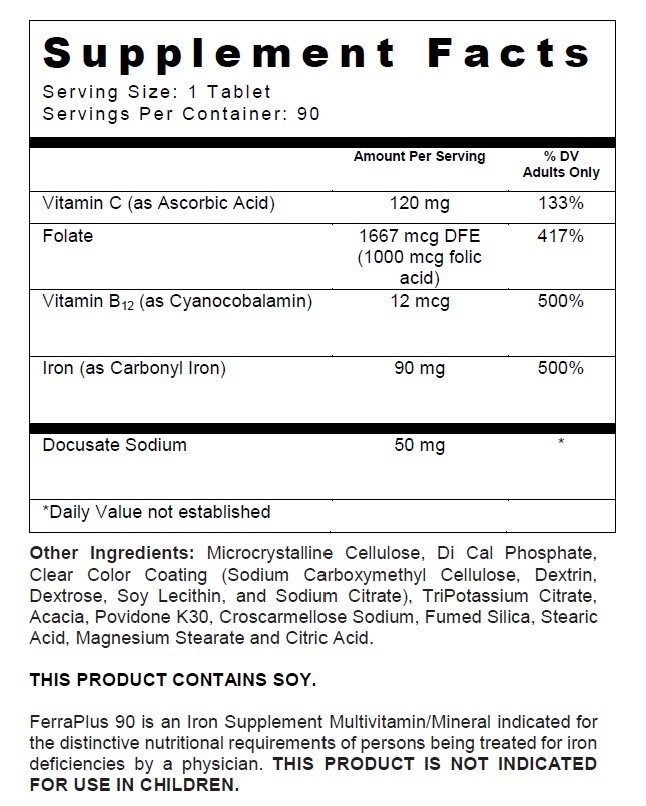
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| imprint | ||
| scoring | 1 | |
| shape | ||
| size (solid drugs) | 15 mm | |
| Labeler - Trigen Laboratories, LLC (830479668) |
More about multivitamin with iron
- Check interactions
- Compare alternatives
- Reviews (30)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: iron products
- En español
Patient resources
Professional resources
Other brands
Dialyvite, Integra Plus, EnLyte, Chromagen, ... +12 more