Nephron FA: Package Insert / Prescribing Info
Package insert / product label
Generic name: iron, ascorbic acid, niacinamide, pyridoxine hydrochloride, pantothenic acid, riboflavin, thiamine, folic acid, biotin and cobalamin
Dosage form: tablet, coated
Drug classes: Iron products, Vitamin and mineral combinations
Medically reviewed by Drugs.com. Last updated on Jul 7, 2025.
Nephron FA Description
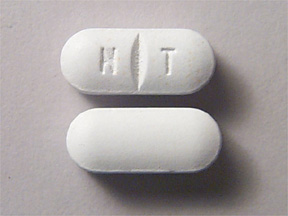
Nephron FA is a prescription folic acid supplement with additional nutrients for kidney dialysis patients. Nephron FA is an elongated, white, film coated tablet imprinted with NT and scored on one side, plain on the other.
Precautions
Folic Acid may obscure pernicious anemia or produce remission while neurologic progress may continue.
Related/similar drugs
Nephron FA Dosage and Administration
One tablet daily taken away from meals, or as directed by the physician.
Package Label/Principal Display Panel
Nephron FA label
59528-4456-1
Rx Only
Nephron FA
Vitamin/Mineral Supplement
100 tablets

NEPHRON FA
mineral/vitamin supplement tablet, coated |
|
|
|
|
|
|
|
|
|
|
|
More about Nephron-FA (multivitamin with iron)
Patient resources
Professional resources
Other brands
Dialyvite, Chromagen
Related treatment guides
Medical Disclaimer