Droperidol: Package Insert / Prescribing Info
Package insert / product label
Dosage form: injection, solution
Drug class: Miscellaneous central nervous system agents
Medically reviewed by Drugs.com. Last updated on Mar 10, 2025.
On This Page
WARNING
Cases of QT prolongation and/or torsade de pointes have been reported in patients receiving droperidol at doses at or below recommended doses. Some cases have occurred in patients with no known risk factors for QT prolongation and some cases have been fatal.
Due to its potential for serious proarrhythmic effects and death, droperidol should be reserved for use in the treatment of patients who fail to show an acceptable response to other adequate treatments, either because of insufficient effectiveness or the inability to achieve an effective dose due to intolerable adverse effects from those drugs (see Warnings, Adverse Reactions, Contraindications, and Precautions).
Cases of QT prolongation and serious arrhythmias (e.g., torsade de pointes) have been reported in patients treated with droperidol. Based on these reports, all patients should undergo a 12-lead ECG prior to administration of droperidol to determine if a prolonged QT interval (i.e., QTc greater than 440 msec for males or 450 msec for females) is present. If there is a prolonged QT interval, droperidol should NOT be administered. For patients in whom the potential benefit of droperidol treatment is felt to outweigh the risks of potentially serious arrhythmias, ECG monitoring should be performed prior to treatment and continued for 2-3 hours after completing treatment to monitor for arrhythmias.
Droperidol is contraindicated in patients with known or suspected QT prolongation, including patients with congenital long QT syndrome.
Droperidol should be administered with extreme caution to patients who may be at risk for development of prolonged QT syndrome (e.g., congestive heart failure, bradycardia, use of a diuretic, cardiac hypertrophy, hypokalemia, hypomagnesemia, or administration of other drugs known to increase the QT interval). Other risk factors may include age over 65 years, alcohol abuse, and use of agents such as benzodiazepines, volatile anesthetics, and I.V. opiates. Droperidol should be initiated at a low dose and adjusted upward, with caution, as needed to achieve the desired effect.
Droperidol Description
Droperidol Injection, USP is a sterile, nonpyrogenic solution of droperidol in water for injection for intravenous or intramuscular injection. Each mL contains droperidol 2.5 mg. Contains lactic acid to adjust pH. pH is 3.4 (3.0 to 3.8).
The solution contains no bacteriostat, antimicrobial agent or added buffer and is intended only for use as a single-dose injection. Discard unused portion.
Droperidol is a neuroleptic (tranquilizer) agent chemically designated as 1-[1-[3-(p-Fluorobenzoyl) propyl]-1,2,3,6-tetrahydro-4-pyridyl]-2-benzimidazolinone with a molecular weight of 379.43.
It has the following structural formula:
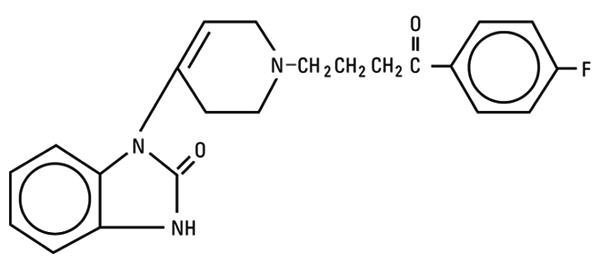
Droperidol - Clinical Pharmacology
Droperidol produces marked tranquilization and sedation. It allays apprehension and provides a state of mental detachment and indifference while maintaining a state of reflex alertness.
Droperidol produces an antiemetic effect as evidenced by the antagonism of apomorphine in dogs. It lowers the incidence of nausea and vomiting during surgical procedures and provides antiemetic protection in the postoperative period.
Droperidol potentiates other CNS depressants. It produces mild alpha-adrenergic blockade, peripheral vascular dilatation and reduction of the pressor effect of epinephrine. It can produce hypotension and decreased peripheral vascular resistance and may decrease pulmonary arterial pressure (particularly if it is abnormally high). It may reduce the incidence of epinephrine-induced arrhythmias but it does not prevent other cardiac arrhythmias.
The onset of action of single intramuscular and intravenous doses is from three to ten minutes following administration, although the peak effect may not be apparent for up to thirty minutes. The duration of the tranquilizing and sedative effects generally is two to four hours, although alteration of alertness may persist for as long as twelve hours.
Indications and Usage for Droperidol
Droperidol injection is indicated to reduce the incidence of nausea and vomiting associated with surgical and diagnostic procedures.
Contraindications
Droperidol is contraindicated in patients with known or suspected QT prolongation (i.e., QTc interval greater than 440 msec for males or 450 msec for females). This would include patients with congenital long QT syndrome.
Droperidol is contraindicated in patients with known hypersensitivity to the drug.
Droperidol is not recommended for any use other than for the treatment of perioperative nausea and vomiting in patients for whom other treatments are ineffective or inappropriate (see WARNINGS).
Warnings
Droperidol should be administered with extreme caution in the presence of risk factors for development of prolonged QT syndrome, such as: 1) clinically significant bradycardia (less than 50 bpm), 2) any clinically significant cardiac disease, 3) treatment with Class I and Class III antiarrhythmics, 4) treatment with monoamine oxidase inhibitors (MAOI’s), 5) concomitant treatment with other drug products known to prolong the QT interval (see PRECAUTIONS, Drug Interactions), and 6) electrolyte imbalance, in particular hypokalemia and hypomagnesemia, or concomitant treatment with drugs (e.g., diuretics) that may cause electrolyte imbalance.
Effects on Cardiac Conduction:
A dose-dependent prolongation of the QT interval was observed within 10 minutes of droperidol administration in a study of 40 patients without known cardiac disease who underwent extracranial head and neck surgery. Significant QT prolongation was observed at all three dose levels evaluated, with 0.1, 0.175, and 0.25 mg/kg associated with prolongation of median QTc by 37, 44, and 59 msec, respectively.
Cases of QT prolongation and serious arrhythmias (e.g., torsade de pointes, ventricular arrhythmias, cardiac arrest, and death) have been observed during post-marketing treatment with droperidol. Some cases have occurred in patients with no known risk factors and at doses at or below recommended doses. There has been at least one case of nonfatal torsade de pointes confirmed by rechallenge.
Based on these reports, all patients should undergo a 12-lead ECG prior to administration of droperidol to determine if a prolonged QT interval (i.e., QTc greater than 440 msec for males or 450 msec for females) is present. If there is a prolonged QT interval, droperidol should NOT be administered. For patients in whom the potential benefit of droperidol treatment is felt to outweigh the risks of potentially serious arrhythmias, ECG monitoring should be performed prior to treatment and continued for 2-3 hours after completing treatment to monitor for arrhythmias.
FLUIDS AND OTHER COUNTERMEASURES TO MANAGE HYPOTENSION SHOULD BE READILY AVAILABLE.
As with other CNS depressant drugs, patients who have received droperidol should have appropriate surveillance.
It is recommended that opioids, when required, initially be used in reduced doses.
As with other neuroleptic agents, very rare reports of neuroleptic malignant syndrome (altered consciousness, muscle rigidity and autonomic instability) have occurred in patients who have received droperidol.
Since it may be difficult to distinguish neuroleptic malignant syndrome from malignant hyperpyrexia in the perioperative period, prompt treatment with dantrolene should be considered if increases in temperature, heart rate or carbon dioxide production occur.
Precautions
General: The initial dose of droperidol should be appropriately reduced in elderly, debilitated and other poor-risk patients. The effect of the initial dose should be considered in determining incremental doses.
Certain forms of conduction anesthesia, such as spinal anesthesia and some peridural anesthetics, can alter respiration by blocking intercostal nerves and can cause peripheral vasodilatation and hypotension because of sympathetic blockade. Through other mechanisms (see CLINICAL PHARMACOLOGY), droperidol can also alter circulation. Therefore, when droperidol is used to supplement these forms of anesthesia, the anesthetist should be familiar with the physiological alterations involved, and be prepared to manage them in the patients elected for these forms of anesthesia.
If hypotension occurs, the possibility of hypovolemia should be considered and managed with appropriate parenteral fluid therapy. Repositioning the patient to improve venous return to the heart should be considered when operative conditions permit. It should be noted that in spinal and peridural anesthesia, tilting the patient into a head-down position may result in a higher level of anesthesia than is desirable, as well as impair venous return to the heart. Care should be exercised in the moving and positioning of patients because of a possibility of orthostatic hypotension. If volume expansion with fluids plus these other countermeasures do not correct the hypotension, then the administration of pressor agents other than epinephrine should be considered. Epinephrine may paradoxically decrease the blood pressure in patients treated with droperidol due to the alpha-adrenergic blocking action of droperidol.
Since droperidol may decrease pulmonary arterial pressure, this fact should be considered by those who conduct diagnostic or surgical procedures where interpretation of pulmonary arterial pressure measurements might determine final management of the patient.
Vital signs and ECG should be monitored routinely.
When the EEG is used for postoperative monitoring, it may be found that the EEG pattern returns to normal slowly.
Impaired Hepatic or Renal Function: Droperidol should be administered with caution to patients with liver and kidney dysfunction because of the importance of these organs in the metabolism and excretion of drugs.
Pheochromocytoma: In patients with diagnosed/ suspected pheochromocytoma, severe hypertension and tachycardia have been observed after the administration of droperidol.
Drug Interactions:
Potentially Arrhythmogenic Agents: Any drug known to have the potential to prolong the QT interval should not be used together with droperidol. Possible pharmacodynamic interactions can occur between droperidol and potentially arrhythmogenic agents such as class I or III antiarrhythmics, antihistamines that prolong the QT interval, antimalarials, calcium channel blockers, neuroleptics that prolong the QT interval, and antidepressants.
Caution should be used when patients are taking concomitant drugs known to induce hypokalemia or hypomagnesemia as they may precipitate QT prolongation and interact with droperidol. These would include diuretics, laxatives and supraphysiological use of steroid hormones with mineralocorticoid potential.
CNS Depressant Drugs: Other CNS depressant drugs (e.g., barbiturates, tranquilizers, opioids and general anesthetics) have additive or potentiating effects with droperidol. Following the administration of droperidol, the dose of other CNS depressant drugs should be reduced.
Carcinogenesis, Mutagenesis, Impairment of Fertility: No carcinogenicity studies have been carried out with droperidol. The micronucleus test in female rats revealed no mutagenic effects in single oral doses as high as 160 mg/kg. An oral study in rats (Segment I) revealed no impairment of fertility in either males or females at 0.63, 2.5 and 10 mg/kg doses (approximately 2, 9 and 36 times maximum recommended human I.V./I.M. dosage).
Pregnancy — Category C: Droperidol administered intravenously has been shown to cause a slight increase in mortality of the newborn rat at 4.4 times the upper human dose. At 44 times the upper human dose, mortality rate was comparable to that for control animals. Following intramuscular administration, increased mortality of the offspring at 1.8 times the upper human dose is attributed to CNS depression in the dams who neglected to remove placentae from their offspring. Droperidol has not been shown to be teratogenic in animals. There are no adequate and well-controlled studies in pregnant women. Droperidol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery: There are insufficient data to support the use of droperidol in labor and delivery. Therefore, such use is not recommended.
Adverse Reactions/Side Effects
QT interval prolongation, torsade de pointes, cardiac arrest, and ventricular tachycardia have been reported in patients treated with droperidol. Some of these cases were associated with death. Some cases occurred in patients with no known risk factors, and some were associated with droperidol doses at or below recommended doses.
Physicians should be alert to palpitations, syncope, or other symptoms suggestive of episodes of irregular cardiac rhythm in patients taking droperidol and promptly evaluate such cases (see WARNINGS, Effects on Cardiac Conduction).
The most common somatic adverse reactions reported to occur with droperidol are mild to moderate hypotension and tachycardia, but these effects usually subside without treatment. If hypotension occurs and is severe or persists, the possibility of hypovolemia should be considered and managed with appropriate parenteral fluid therapy.
The most common behavioral adverse effects of droperidol include dysphoria, postoperative drowsiness, restlessness, hyperactivity and anxiety, which can either be the result of an inadequate dosage (lack of adequate treatment effect) or of an adverse drug reaction (part of the symptom complex of akathisia).
Care should be taken to search for extrapyramidal signs and symptoms (dystonia, akathisia, oculogyric crisis) to differentiate these different clinical conditions. When extrapyramidal symptoms are the cause, they can usually be controlled with anticholinergic agents.
Postoperative hallucinatory episodes (sometimes associated with transient periods of mental depression) have also been reported.
Other less common reported adverse reactions include anaphylaxis, dizziness, chills and/or shivering, laryngospasm and bronchospasm.
Elevated blood pressure, with or without pre-existing hypertension, has been reported following administration of droperidol combined with fentanyl citrate or other parenteral analgesics. This might be due to unexplained alterations in sympathetic activity following large doses; however, it is also frequently attributed to anesthetic or surgical stimulation during light anesthesia.
Related/similar drugs
Overdosage
Manifestations: The manifestations of droperidol overdosage are an extension of its pharmacologic actions and may include QT prolongation and serious arrhythmias (e.g. torsade de pointes) (see BOX WARNING, WARNINGS, and PRECAUTIONS).
Treatment: In the presence of hypoventilation or apnea, oxygen should be administered and respiration should be assisted or controlled as indicated. A patent airway must be maintained; an oropharyngeal airway or endotracheal tube might be indicated. The patient should be carefully observed for 24 hours; body warmth and adequate fluid intake should be maintained. If hypotension occurs and is severe or persists, the possibility of hypovolemia should be considered and managed with appropriate parenteral fluid therapy. (See PRECAUTIONS).
If significant extrapyramidal reactions occur, in the context of an overdose, an anticholinergic should be administered.
The intravenous Median Lethal Dose is 20 ― 43 mg/kg in mice; 30 mg/kg in rats; and 25 mg/kg in dogs and 11 ― 13 mg/kg in rabbits. The intramuscular Median Lethal Dose of droperidol is 195 mg/kg in mice; 104 ― 110 mg/kg in rats; 97 mg/kg in rabbits and 200 mg/kg in guinea pigs.
Droperidol Dosage and Administration
Dosage should be individualized. Some of the factors to be considered in determining dose are age, body weight, physical status, underlying pathological condition, use of other drugs, the type of anesthesia to be used, and the surgical procedure involved.
Vital signs and ECG should be monitored routinely.
Adult Dosage: The maximum recommended initial dose of droperidol is 2.5 mg I.M. or slow I.V. Additional 1.25 mg doses of droperidol may be administered to achieve the desired effect. However, additional doses should be administered with caution, and only if the potential benefit outweighs the potential risk.
Pediatric Dosage: For children two to 12 years of age, the maximum recommended initial dose is 0.1 mg/kg, taking into account the patient’s age and other clinical factors. However, additional doses should be administered with caution, and only if the potential benefit outweighs the potential risk.
See WARNINGS and PRECAUTIONS for use of droperidol with other CNS depressants and in patients with altered response.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If such abnormalities are observed, the drug should not be administered.
How is Droperidol supplied
Droperidol Injection, USP 2.5 mg/mL is supplied in 2 mL (5 mg) single-dose ampuls packaged in cartons of ten (List No. 1187).
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Protect from light.
References
-
Saarnivaara L, Klemola UM, Lindgren L, et al. QT interval of the ECG, heart rate and arterial pressure using propofol, methohexital or midazolam for induction of anesthesia. Acta Anaesthesiol Scand 1990: 34: 276-81.
-
Schmeling WT, Warltier DC, McDonald DJ, et al. Prolongation of the QT interval by enflurane, isoflurane, and halothane in humans. Anesth Analg 1991:72:137-44.
-
Späth G. Torsade des pointe oder die andere Ursache des plötz-lichen Herztodes. Wien: Ueberreuter, 1998.
-
Riley DC, Schmeling WT, Al-Wathiqui MH, et al. Prolongation of the QT-interval by volatile anesthetics in chronically instrumented dogs. Anesth Analg 1988: 67: 741-9.
-
McConachie I, Keaveny JP, Healy TF, et al. Effects of anaesthesia on the QT-interval. Br J Anaesth 1989: 63: 558-60.
-
Lawrence KR, Nasraway SA. Conduction disturbances associated with administration of butyrophenone antipsychotics in the critically ill: a review of the literature. Pharmacotherapy 1997: 17(3): 531-7.
-
Lischke V, Behne M, Doelken P, et al. Droperidol causes a dose-dependent prolongation of the QT interval. Anesth Analg 1994: 79: 983-6.
Revised: October, 2004
©Hospira 2004 EN-0531 Printed in USA
HOSPIRA, INC., LAKE FOREST, IL 60045 USA
PRINCIPAL DISPLAY PANEL - 2 mL Ampul Label
2 mL
NDC 0409-1187-11
Droperidol Inj., USP
5 mg/2 mL (2.5 mg/mL)
Rx only
I.V. or I.M. Use. Protect from light.
RL-7358
Distributed by
Hospira, Inc.,
Lake Forest, IL
60045 USA

PRINCIPAL DISPLAY PANEL - Ten 2 mL Ampul Carton Label
Ten 2 mL Single-dose Ampuls
Rx only
NDC 0409-1187-01
Contains 10 of NDC 0409-1187-11
Droperidol Injection, USP
5 mg/2 mL (2.5 mg/mL)
Protect from light.
Keep ampuls in tray until time of use.
Each mL contains droperidol 2.5 mg. Contains lactic acid for pH adjustment.
pH 3.4 (3.0 to 3.8).
FOR INTRAVENOUS OR INTRAMUSCULAR USE.
Usual dosage: See Insert. Store at 20 to 25°C (68 to 77°F).
[See USP Controlled Room Temperature.]
Distributed by Hospira, Inc.,
Lake Forest, IL 60045 USA
RL-7359
| For your convenience in recording use | |||
| INITIAL/DATE | INITIAL/DATE | ||
| 1. | 6. | ||
| 2. | 7. | ||
| 3. | 8. | ||
| 4. | 9. | ||
| 5. | 10. | ||
| DROPERIDOL
droperidol injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hospira, Inc. (141588017) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hospira, Inc. | 093132819 | ANALYSIS(0409-1187) , LABEL(0409-1187) , MANUFACTURE(0409-1187) , PACK(0409-1187) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hospira, Inc. | 827731089 | ANALYSIS(0409-1187) | |
More about droperidol
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (45)
- Side effects
- Dosage information
- During pregnancy
- Drug class: miscellaneous central nervous system agents
- Breastfeeding
- En español
