Daybue Oral Solution: Package Insert / Prescribing Info
Package insert / product label
Generic name: trofinetide
Dosage form: oral solution
Drug class: Miscellaneous central nervous system agents
Medically reviewed by Drugs.com. Last updated on Aug 25, 2025.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
DAYBUE® (trofinetide) oral solution
Initial U.S. Approval: 2023
Recent Major Changes
Indications and Usage for Daybue Oral Solution
DAYBUE is indicated for the treatment of Rett syndrome in adults and pediatric patients 2 years of age and older. (1)
Daybue Oral Solution Dosage and Administration
- Recommended dosage is twice daily, morning and evening, according to patient weight. DAYBUE can be given with or without food. (2.1)
| Patient Weight | DAYBUE Dosage | DAYBUE Volume |
|---|---|---|
| 9 kg to less than 12 kg | 5,000 mg twice daily | 25 mL twice daily |
| 12 kg to less than 20 kg | 6,000 mg twice daily | 30 mL twice daily |
| 20 kg to less than 35 kg | 8,000 mg twice daily | 40 mL twice daily |
| 35 kg to less than 50 kg | 10,000 mg twice daily | 50 mL twice daily |
| 50 kg or more | 12,000 mg twice daily | 60 mL twice daily |
Dosage Forms and Strengths
- Oral solution: 200 mg/mL (3)
Contraindications
None. (4)
Warnings and Precautions
- Diarrhea: Most patients experience diarrhea during treatment with DAYBUE. Advise patients to stop laxatives before starting DAYBUE. If diarrhea occurs, patients should start antidiarrheal treatment, increase oral fluids, and notify their healthcare provider. Interrupt, reduce dose, or discontinue DAYBUE if severe diarrhea occurs or if dehydration is suspected. (2.3, 5.1)
- Weight Loss: Weight loss may occur in patients treated with DAYBUE. Monitor weight and interrupt, reduce dose, or discontinue DAYBUE if significant weight loss occurs. (2.3, 5.2)
- Vomiting: Aspiration and aspiration pneumonia have occurred after vomiting in patients treated with DAYBUE. Interrupt, reduce dose, or discontinue DAYBUE if vomiting is severe or occurs despite medical management. (2.4, 5.3)
Adverse Reactions/Side Effects
The most common adverse reactions (that occurred in at least 10% of DAYBUE-treated patients and at least 2% greater than in placebo) were diarrhea and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Acadia Pharmaceuticals Inc. at 1-844-422-2342 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Orally administered CYP3A and/or P-gp sensitive substrates for which a small change in substrate plasma concentration may lead to serious adverse reactions: closely monitor for adverse reactions with concomitant use. (7.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2025
Full Prescribing Information
1. Indications and Usage for Daybue Oral Solution
DAYBUE is indicated for the treatment of Rett syndrome in adults and pediatric patients 2 years of age and older.
2. Daybue Oral Solution Dosage and Administration
2.1 Dosing Information
Administer DAYBUE orally twice daily, in the morning and evening, according to patient weight as shown in Table 1. DAYBUE can be taken with or without food.
| Patient Weight | DAYBUE Dosage | DAYBUE Volume |
|---|---|---|
| 9 kg to less than 12 kg | 5,000 mg twice daily | 25 mL twice daily |
| 12 kg to less than 20 kg | 6,000 mg twice daily | 30 mL twice daily |
| 20 kg to less than 35 kg | 8,000 mg twice daily | 40 mL twice daily |
| 35 kg to less than 50 kg | 10,000 mg twice daily | 50 mL twice daily |
| 50 kg or more | 12,000 mg twice daily | 60 mL twice daily |
2.2 Administration Information
Administer DAYBUE orally or via gastrostomy (G) tube; doses administered via gastrojejunal (GJ) tubes must be administered through the G-port.
A calibrated measuring device, such as an oral syringe or oral dosing cup, should be obtained from the pharmacy to measure and deliver the prescribed dose accurately. A household measuring cup is not an adequate measuring device.
Discard any unused DAYBUE oral solution after 14 days of first opening the bottle [see How Supplied/Storage and Handling (16.2)].
2.3 Dose Modification for Diarrhea or Weight Loss
Advise patients to stop laxatives before starting DAYBUE. Interrupt, reduce dose, or discontinue DAYBUE if severe diarrhea occurs, if dehydration is suspected, or if significant weight loss occurs [see Warnings and Precautions (5.1, 5.2)].
2.4 Dose Modification for Vomiting After Administration
If vomiting occurs after DAYBUE administration, an additional dose should not be taken. Instead, continue with the next scheduled dose. Interrupt, reduce dose, or discontinue DAYBUE if vomiting is severe or occurs despite medical management [see Warnings and Precautions (5.3)].
2.5 Dosage Recommendations in Patients With Renal Impairment
No dosage adjustment is recommended for patients with mild renal impairment (estimated glomerular filtration rate [eGFR] 60 to 89 mL/min for adult patients or 60 to 89 mL/min/1.73 m2 for pediatric patients). The recommended dosage of DAYBUE for patients with moderate renal impairment (eGFR 30 to 59 mL/min for adult patients or 30 to 59 mL/min/1.73 m2 for pediatric patients) is described in Table 2 [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)]. DAYBUE is not recommended for patients with severe renal impairment (eGFR less than 30 mL/min for adult patients or less than 30 mL/min/1.73 m2 for pediatric patients).
| Patient Weight | DAYBUE Dosage | DAYBUE Volume |
|---|---|---|
| 9 kg to less than 12 kg | 2,500 mg twice daily | 12.5 mL twice daily |
| 12 kg to less than 20 kg | 3,000 mg twice daily | 15 mL twice daily |
| 20 kg to less than 35 kg | 4,000 mg twice daily | 20 mL twice daily |
| 35 kg to less than 50 kg | 5,000 mg twice daily | 25 mL twice daily |
| 50 kg or more | 6,000 mg twice daily | 30 mL twice daily |
3. Dosage Forms and Strengths
Trofinetide oral solution: 200 mg/mL of a pink to red, strawberry flavored solution.
5. Warnings and Precautions
5.1 Diarrhea
In Study 1 [see Clinical Studies (14)] and in long-term studies, 85% of patients treated with DAYBUE experienced diarrhea. In those treated with DAYBUE, 49% either had persistent diarrhea or recurrence after resolution despite dose interruptions, reductions, or concomitant antidiarrheal therapy. Diarrhea severity was of mild or moderate severity in 96% of cases. In Study 1, antidiarrheal medication was used in 51% of patients treated with DAYBUE.
Advise patients to stop laxatives before starting DAYBUE. If diarrhea occurs, patients should notify their healthcare provider, consider starting antidiarrheal treatment, and monitor hydration status and increase oral fluids, if needed. Interrupt, reduce dose, or discontinue DAYBUE if severe diarrhea occurs or if dehydration is suspected [see Dosage and Administration (2.3)].
5.2 Weight Loss
In Study 1, 12% of patients treated with DAYBUE experienced weight loss of greater than 7% from baseline, compared to 4% of patients who received placebo. In long-term studies, 2.2% of patients discontinued treatment with DAYBUE due to weight loss.
Monitor weight and interrupt, reduce dose, or discontinue DAYBUE if significant weight loss occurs [see Dosage and Administration (2.3)].
5.3 Vomiting
In Study 1, vomiting occurred in 29% of patients treated with DAYBUE and in 12% of patients who received placebo [see Adverse Reactions (6.1)].
Patients with Rett syndrome are at risk for aspiration and aspiration pneumonia. Aspiration and aspiration pneumonia have been reported following vomiting in patients being treated with DAYBUE. Interrupt, reduce dose, or discontinue DAYBUE if vomiting is severe or occurs despite medical management [see Dosage and Administration (2.4)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in labeling:
- Diarrhea [see Warnings and Precautions (5.1)]
- Weight Loss [see Warnings and Precautions (5.2)]
- Vomiting [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In controlled and uncontrolled trials in patients with Rett syndrome, 260 patients ages 2 to 40 years were treated with DAYBUE, including 109 patients treated for more than 6 months, 69 patients treated for more than 1 year, and 4 patients treated for more than 2 years.
Adult and Pediatric Patients With Rett Syndrome 5 Years of Age and Older
The safety of DAYBUE was evaluated in a randomized, double-blind, placebo-controlled, 12-week study of patients with Rett syndrome (Study 1) [see Clinical Studies (14)]. In Study 1, 93 patients received DAYBUE and 94 patients received placebo. All patients were female, 92% were White, and the mean age was 11 years (range 5 to 20 years).
Adverse Reactions Leading to Discontinuation of Treatment
Eighteen patients (19%) receiving DAYBUE had adverse reactions that led to withdrawal from the study. The most common adverse reaction leading to discontinuation of treatment with DAYBUE was diarrhea (15%).
Common Adverse Reactions
Adverse reactions that occurred in Study 1 in at least 5% of patients treated with DAYBUE and were at least 2% more frequent than in patients on placebo are presented in Table 3.
| Adverse Reaction | DAYBUE (N=93) % | Placebo (N=94) % |
|---|---|---|
| Diarrhea | 82 | 20 |
| Vomiting | 29 | 12 |
| Fever | 9 | 4 |
| Seizure | 9 | 6 |
| Anxiety | 8 | 1 |
| Decreased appetite | 8 | 2 |
| Fatigue | 8 | 2 |
| Nasopharyngitis | 5 | 1 |
Pediatric Patients With Rett Syndrome 2 to 4 Years of Age
In an open-label study in pediatric patients 2 to 4 years of age with Rett syndrome, a total of 13 patients received DAYBUE for at least 12 weeks and 9 patients received DAYBUE for at least 6 months. Adverse reactions in pediatric patients 2 to 4 years of age treated with DAYBUE were similar to those reported in adult and pediatric patients 5 years of age and older with Rett syndrome in Study 1.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of DAYBUE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Aspiration and aspiration pneumonia secondary to vomiting [see Warnings and Precautions (5.3)].
Related/similar drugs
7. Drug Interactions
7.1 Effect of DAYBUE on Other Drugs
CYP3A and/or P-gp Substrates
Closely monitor patients when DAYBUE is administered concomitantly with sensitive CYP3A and/or P-gp substrates where minimal increases in the plasma concentration of these substrates may lead to serious adverse reactions. Trofinetide, a weak inhibitor of CYP3A and an inhibitor of P-gp, increased the plasma concentrations of CYP3A and/or P-gp substrates [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions associated with these substrates.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risks associated with the use of DAYBUE in pregnant women. No adverse developmental effects were observed following oral administration of trofinetide to pregnant animals at doses associated with plasma exposures below those used clinically [see Animal Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Oral administration of trofinetide (0, 150, 450, or 1000 mg/kg twice daily; 0, 300, 900, or 2000 mg/kg/day) to pregnant rats during the period of organogenesis resulted in no adverse effects on embryofetal development. At the highest dose tested, plasma exposure (AUC) was less than that in humans at the maximum recommended human dose (MRHD) of 12,000 mg twice daily (24,000 mg/day).
Oral administration of trofinetide (0, 75, 150, or 300 mg/kg twice daily; 0, 150, 300, or 600 mg/kg/day) to pregnant rabbits during the period of organogenesis resulted in no adverse effects on embryofetal development. At the highest dose tested, plasma exposure (AUC) was less than that in humans at the MRHD.
Oral administration of trofinetide (0, 150, 450, or 1000 mg/kg twice daily; 0, 300, 900, or 2000 mg/kg/day) to rats throughout pregnancy and lactation resulted in no adverse effects on pre- and postnatal development. At the highest dose tested, plasma exposure (AUC) was less than that in humans at the MRHD.
8.2 Lactation
Risk Summary
There is no information regarding the presence of trofinetide or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for DAYBUE and any potential adverse effects on the breastfed infant from DAYBUE or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of DAYBUE for the treatment of Rett syndrome have been established in pediatric patients aged 2 years and older. The safety and effectiveness of DAYBUE for the treatment of Rett syndrome in pediatric patients 5 years of age and older was established in a randomized, double-blind, placebo-controlled, 12-week study (Study 1), which included 108 pediatric patients age 5 to less than 12 years of age and 47 pediatric patients age 12 to less than 17 years of age [see Adverse Reactions (6.1) and Clinical Studies (14)]. Use of DAYBUE in patients 2 to 4 years of age is supported by evidence from Study 1 and pharmacokinetic and safety data in 13 pediatric patients 2 to 4 years of age treated with DAYBUE for 12 weeks [see Dosage and Administration (2.1), Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)].
Safety and effectiveness in pediatric patients less than 2 years of age have not been established.
Juvenile Animal Data
Oral administration of trofinetide (0, 150, 300, or 1000 mg/kg twice daily; 0, 300, 600, or 2000 mg/kg/day) to rats from postnatal day (PND) 13-14 through 28 weeks of age resulted in no adverse effects on growth or neurobehavioral function. Plasma exposures at the highest dose tested were similar to those in pediatric patients at recommended doses.
Oral administration of trofinetide (0, 150, 300, or 1000 mg/kg twice daily; 0, 300, 600, or 2000 mg/kg/day) to juvenile rats for 10 weeks beginning on PND 13-14 resulted in no adverse effects on sexual maturation or reproductive function. Plasma exposures at the highest dose tested were similar to those in pediatric patients at recommended doses.
8.5 Geriatric Use
Clinical studies of DAYBUE did not include patients 65 years of age and older to determine whether or not they respond differently from younger patients. This drug is known to be substantially excreted by the kidney. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function.
8.6 Renal Impairment
Mild renal impairment is not expected to impact the exposure of trofinetide; therefore, dosage adjustment is not necessary. Dosage adjustment of DAYBUE is recommended in patients with moderate renal impairment (adult: eGFR 30 to 59 mL/min; pediatric: eGFR 30 to 59 mL/min/1.73 m2) [see Dosage and Administration (2.5), Clinical Pharmacology (12.3)]. Administration of DAYBUE to patients with severe renal impairment (eGFR less than 30 mL/min for adults or less than 30 mL/min/1.73 m2 for pediatrics) is not recommended.
11. Daybue Oral Solution Description
Trofinetide is designated chemically as (2S)-2-{[(2S)-1-(2-aminoacetyl)-2-methylpyrrolidine-2-carbonyl]amino}pentanedioic acid (IUPAC). Its empirical formula is C13H21N3O6 and its molecular weight is 315.33 g/mol. The chemical structure is:
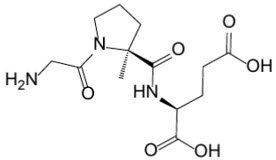
Trofinetide is a white to off-white solid and is freely soluble in water.
DAYBUE is a pink to red, oral solution with each 5 mL containing 1 g of trofinetide (200 mg/mL). The oral solution also contains FD&C Red No. 40, maltitol, methylparaben sodium, propylparaben sodium, purified water, strawberry flavor, and sucralose as inactive ingredients.
12. Daybue Oral Solution - Clinical Pharmacology
12.1 Mechanism of Action
The mechanism by which trofinetide exerts therapeutic effects in patients with Rett syndrome is unknown.
12.3 Pharmacokinetics
Trofinetide exhibits linear kinetics with no time- or dose-dependent effect on pharmacokinetic parameters. Systemic exposure to trofinetide was dose-proportional across the studied dose range. Minimal to no accumulation was observed following multiple-dose administration.
Absorption
The time to maximum drug concentration (Tmax) is about 2 to 3 hours after administration. Based on the mass balance study, at least 84% of the administered dose was absorbed following oral administration of 12,000 mg trofinetide.
Effect of Food
Coadministration of DAYBUE with a high-fat meal had no impact on the total exposure (AUC0-inf) of trofinetide and reduced the peak plasma concentration (Cmax) by approximately 20% [see Dosage and Administration (2.1)].
Distribution
Following oral administration, the apparent volume of distribution of trofinetide in adult healthy subjects was approximately 80 L. Trofinetide protein binding in human plasma is less than 6%.
Elimination
The effective elimination half-life of orally administered trofinetide in healthy subjects is about 1.5 hours.
Specific Populations
Pediatric Patients
The drug exposure of trofinetide in pediatric patients ages 2 to 4 years of age is similar to children older than 4 years and adults when following the recommended dosage [see Dosage and Administration (2.1)].
Patients with Renal Impairment
Based on population PK analysis of clinical trials data, patients with mild renal impairment (eGFR 60 to 89 mL/min/1.73 m2) showed no significant impact on the exposure of trofinetide compared to patients with normal renal function. Based on a renal impairment study in adult subjects, the effect of moderate renal impairment (eGFR 30 to 59 mL/min) increases the exposure (AUC0-inf) of trofinetide approximately 80% compared to patients with normal renal function administered the same dose [see Dosage and Administration (2.5)]. The effect of severe renal impairment on the exposure of trofinetide has not been investigated [see Use in Specific Populations (8.6)].
Drug Interaction Studies
Clinical Studies
CYP3A and/or P-gp Substrates:
Coadministration of trofinetide 12,000 mg twice daily with 4 mg of loperamide (a moderately sensitive CYP3A substrate and a P-gp substrate) increased the AUC of loperamide by 1.73-fold and the Cmax by 1.95-fold [see Drug Interactions (7.1)]. Administration of trofinetide 2 hours prior to loperamide increased the AUC of loperamide by 1.22-fold and the Cmax by 1.44-fold.
In Vitro
Trofinetide is not a substrate of CYP450 enzymes, uridine diphosphate glucuronosyltransferase (UGT), or major drug transporters.
Cytochrome P450 (CYP450) Enzymes:
Trofinetide inhibits CYP3A [see Drug Interactions (7.1)]. Trofinetide inhibits CYP1A2, 2B6, 2C8, 2C19, and 2D6, but is not expected to result in clinically significant drug interactions. Trofinetide does not inhibit CYP2C9.
Transporter Systems:
Trofinetide inhibits P-gp [see Drug Interactions (7.1)], BCRP, and BSEP. Trofinetide inhibits OAT1, OCT2, OATP1B1, OATP1B3, MATE1, and MATE2-K, but is not expected to result in clinically significant drug interactions. Trofinetide does not inhibit OAT3.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Studies to evaluate the carcinogenic potential of trofinetide have not been conducted.
Mutagenesis
Trofinetide was negative in in vitro (bacterial reverse mutation, chromosomal aberration in Chinese hamster ovary cells) and in vivo (mouse micronucleus) assays.
Impairment of Fertility
Oral administration of trofinetide (0, 150, 450, or 1000 mg/kg twice daily; 0, 300, 900, or 2000 mg/kg/day) to male and female rats prior to and throughout mating and continuing in females through gestation day 7 resulted in no adverse effects on fertility or reproductive function. Plasma exposures at the highest dose tested were less than that in humans at the maximum recommended human dose of 12,000 mg/dose (24,000 mg/day).
14. Clinical Studies
The efficacy of DAYBUE for the treatment of Rett syndrome was established in a 12-week randomized, double-blind, placebo-controlled study in patients with Rett syndrome 5 to 20 years of age (Study 1; NCT04181723).
Patients (N=187) had a diagnosis of typical Rett syndrome according to the Rett Syndrome Diagnostic Criteria with a documented disease-causing mutation in the MECP2 gene. Patients were randomized to receive DAYBUE (N=93) or matching placebo (N=94) for 12 weeks. The DAYBUE dosage was based on patient weight to achieve similar exposure in all patients [see Dosage and Administration (2.1)].
The co-primary efficacy measures were change from baseline after 12 weeks of treatment in the total score of the Rett Syndrome Behaviour Questionnaire (RSBQ) and the Clinical Global Impression-Improvement (CGI-I) score. The RSBQ is a 45-item rating scale completed by the caregiver that assesses a range of symptoms of Rett syndrome (breathing, hand movements or stereotypies, repetitive behaviors, night-time behaviors, vocalizations, facial expressions, eye gaze, and mood). Each item is scored as 0 (not true), 1 (somewhat or sometimes true), or 2 (very true or often true), with a maximum possible score of 90 points. Lower scores reflect lesser severity in signs and symptoms of Rett syndrome. The CGI-I is rated by clinicians to assess whether a patient has improved or worsened on a 7-point scale (1=very much improved to 7=very much worse) in which a decrease in score indicates improvement.
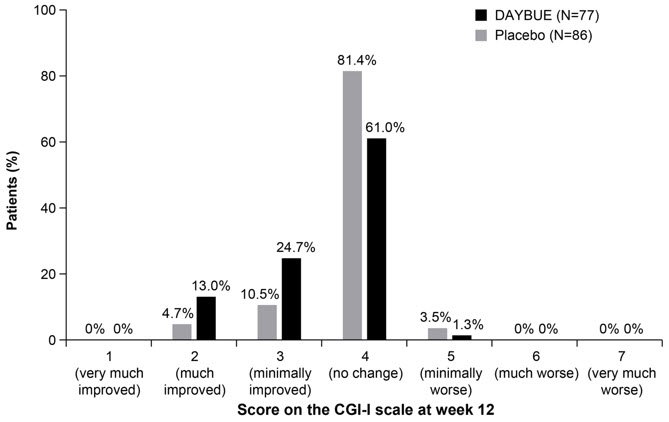
Treatment with DAYBUE demonstrated a statistically significant difference in favor of DAYBUE as compared to placebo on the co-primary efficacy endpoints, the change from baseline in RSBQ total score, and the CGI-I score at week 12 (Table 4, Figure 1, and Figure 2).
| Mean Baseline Score (SE) | Mean Week 12 Score (SE) | LS Mean Change from Baseline to Week 12 (SE) | DAYBUE-Placebo Treatment Difference, LS Mean (95% CI)* | p-value | ||
|---|---|---|---|---|---|---|
| CI=confidence interval; LS mean=least-squares mean; SE=standard error | ||||||
|
||||||
| RSBQ | DAYBUE | 43.7 (1.21) | 39.9 (1.38) | -4.9 (0.94) | -3.2 (-5.7, -0.6) | 0.018 |
| Placebo | 44.5 (1.26) | 42.8 (1.42) | -1.7 (0.90) | |||
| CGI-I | DAYBUE | -- | 3.5 (0.08) | -- | -0.3 (-0.5, -0.1) | 0.003 |
| Placebo | -- | 3.8 (0.06) | ||||
Figure 1 Change From Baseline in RSBQ Total Score in Study 1
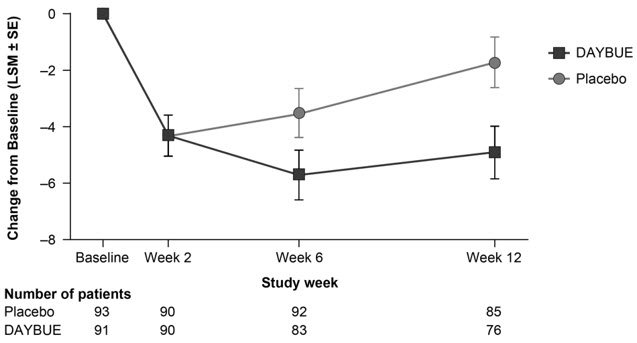
Figure 2 Distribution of CGI-I Scores for Patients Completing Study 1
16. How is Daybue Oral Solution supplied
17. Patient Counseling Information
Advise the caregiver or patient to read the FDA-approved patient labeling (Patient Information).
DAYBUE Administration
Advise the caregiver or patient that DAYBUE may be given orally or via gastrostomy (G) tube; doses administered via gastrojejunal (GJ) tubes must be administered through the G-port. DAYBUE may be taken with or without food [see Dosage and Administration (2.1, 2.2)].
Instruct the caregiver or patient to obtain a calibrated measuring device, such as an oral syringe or oral dosing cup, from the pharmacy to measure and deliver the prescribed dose accurately. A household measuring cup is not an adequate measuring device.
Instruct the caregiver or patient to discard any unused DAYBUE after 14 days of first opening the bottle.
Diarrhea
Advise the caregiver or patient that DAYBUE can cause diarrhea. Instruct the patient to stop taking laxatives before starting DAYBUE. If diarrhea occurs, patients should notify their healthcare provider, consider starting antidiarrheal treatment, and monitor hydration status and increase oral fluids, if needed [see Warnings and Precautions (5.1)].
Weight Loss
Inform the caregiver or patient that DAYBUE may cause weight loss and to notify their healthcare provider if weight loss occurs [see Warnings and Precautions (5.2)].
Vomiting
Advise the caregiver or patient that DAYBUE can cause vomiting and if vomiting occurs after DAYBUE administration, do not take an additional dose, but continue with the next scheduled dose [see Dosage and Administration (2.4)]. Instruct patients to notify their healthcare provider if vomiting does not stop despite medical management [see Warnings and Precautions (5.3)].
Storage
Keep bottles of DAYBUE oral solution upright and refrigerated before and after opening. Do not freeze [see How Supplied/Storage and Handling (16.2)].
Marketed by:
Acadia Pharmaceuticals Inc. San Diego, CA 92130 USA
DAYBUE is a registered trademark of Acadia Pharmaceuticals Inc.
©2025 Acadia Pharmaceuticals Inc. All rights reserved.
| PATIENT INFORMATION DAYBUE® (day-BYOO) (trofinetide) oral solution |
|
|---|---|
| This Patient Information has been approved by the U.S. Food and Drug Administration | Approved 8/2025 |
What is DAYBUE?
|
|
Before taking DAYBUE, tell your healthcare provider about all of your medical conditions, including if you:
Taking DAYBUE with certain medicines may affect the way other medicines work and can cause serious side effects. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
|
How should I take DAYBUE?
|
|
| What are the possible side effects of DAYBUE?
DAYBUE may cause side effects, including:
These are not all the possible side effects of DAYBUE. Tell your healthcare provider if you have any side effects that bother you or do not go away. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store DAYBUE?
|
|
| General information about the safe and effective use of DAYBUE.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use DAYBUE for a condition for which it was not prescribed. Do not give DAYBUE to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about DAYBUE that is written for health professionals. |
|
| What are the ingredients in DAYBUE?
Active ingredient: trofinetide Inactive ingredients: FD&C Red No. 40, maltitol, methylparaben sodium, propylparaben sodium, purified water, strawberry flavor, and sucralose. Marketed by Acadia Pharmaceuticals Inc., San Diego, CA 92130 USA DAYBUE is a registered trademark of Acadia Pharmaceuticals Inc. ©2025 Acadia Pharmaceuticals Inc. All rights reserved. For more information, go to www.daybue.com or call 1-844-422-2342. |
|
| DAYBUE
trofinetide solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Acadia Pharmaceuticals Inc. (963571302) |
More about Daybue (trofinetide)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Drug images
- Side effects
- Dosage information
- During pregnancy
- FDA approval history
- Drug class: miscellaneous central nervous system agents
- Breastfeeding
- En español


