Anesthetic Gel: Package Insert / Prescribing Info
Package insert / product label
Generic name: lidocaine hydrochloride
Dosage form: gel
Drug class: Topical anesthetics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2025.
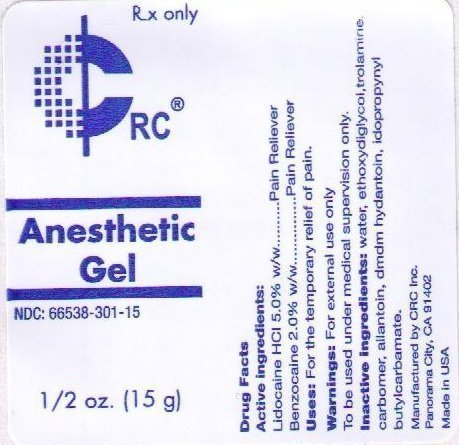
ACTIVE INGREDIENTS:
LIDOCAINE HCL 5.0% W/W
USES: FOR THE TEMPORARY RELIEF OF PAIN.
WARNINGS: FOR EXTERNAL USE ONLY.
TO BE USED UNDER MEDICAL SUPERVISION ONLY.
INACTIVE INGREDIENTS: WATER, ETHOXYDIGLYCOL, TROLAMINE, CARBOMER, ALLANTOIN, DMDM HYDANTOIN, IODOPROPYNYL BUTYLCARBAMATE, ETHYL AMINOBENZOATE.
RX ONLY
CRC
ANESTHETIC GEL
NDC: 66538-301-15
1/2 OZ. (15 ML)
KEEP OUT OF REACH OF CHILDREN.
ANESTHETIC
lidocaine hydrochloride benzocaine gel |
|
|
|
|
|
|
|
|
|
|
|
|
Related/similar drugs
Medical Disclaimer

